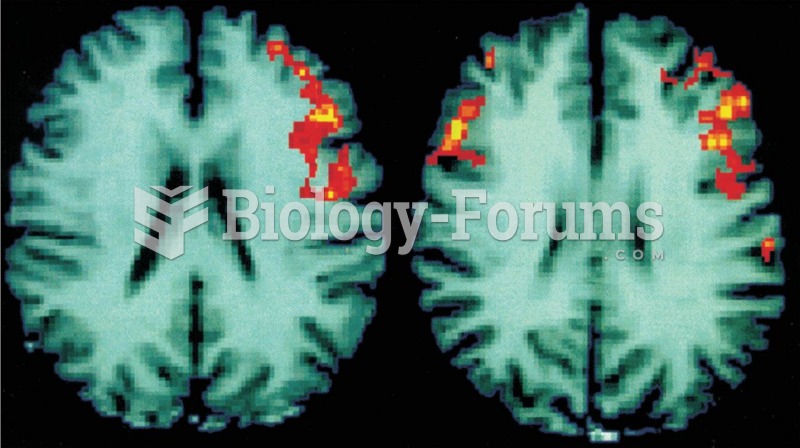
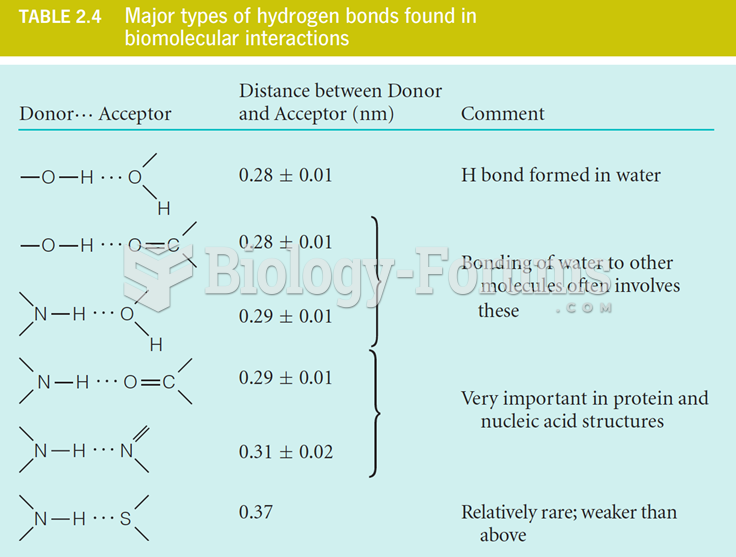
|
|
|
According to animal studies, the typical American diet is damaging to the liver and may result in allergies, low energy, digestive problems, and a lack of ability to detoxify harmful substances.
Cutaneous mucormycosis is a rare fungal infection that has been fatal in at least 29% of cases, and in as many as 83% of cases, depending on the patient's health prior to infection. It has occurred often after natural disasters such as tornados, and early treatment is essential.
The first monoclonal antibodies were made exclusively from mouse cells. Some are now fully human, which means they are likely to be safer and may be more effective than older monoclonal antibodies.
After a vasectomy, it takes about 12 ejaculations to clear out sperm that were already beyond the blocked area.
HIV testing reach is still limited. An estimated 40% of people with HIV (more than 14 million) remain undiagnosed and do not know their infection status.
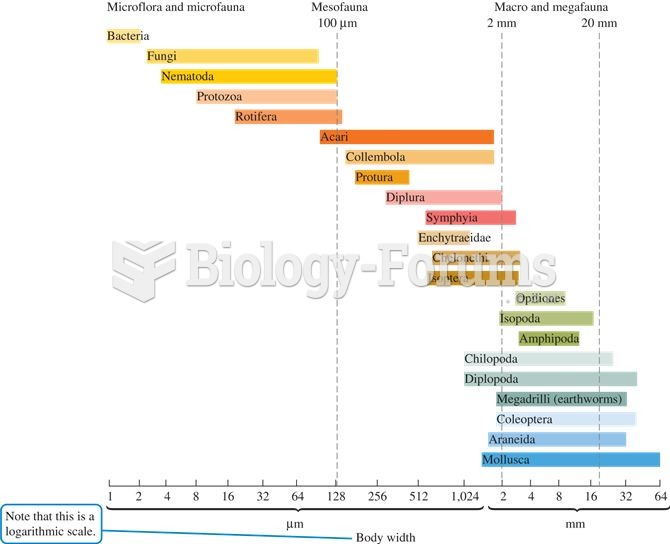 Detritivores are often classified according to their body size as microflora, microfauna, mesofauna,
Detritivores are often classified according to their body size as microflora, microfauna, mesofauna,
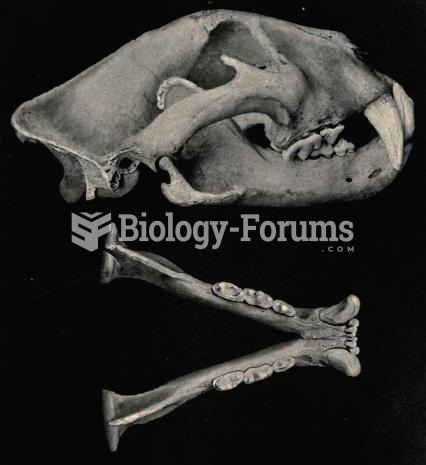 The head of the jaguar is robust and the jaw extremely powerful. The size of jaguars tends to increa
The head of the jaguar is robust and the jaw extremely powerful. The size of jaguars tends to increa
 Risks of infectious disease increase in (a) high-density agricultural populations compared to (b) lo
Risks of infectious disease increase in (a) high-density agricultural populations compared to (b) lo