Answer to Question 1ANS:Answer
should include:
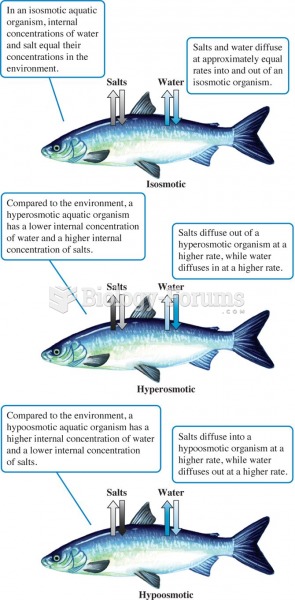
Water's polarity allows it to form hydrogen bonds with other charged molecules. The
partially positive hydrogen atoms attract to the partially negative oxygen atoms of other
water molecules. The tendency of water to stick to other water molecules is called
cohesion. This property creates a skin on the surface called surface tension.
Water also has a propensity to stick to other solids as well. This is called adhesion.
When water molecules begin to attach to other molecules, it can affect the amount of
energy is needed for a phase change.
The hydrogen bonds add strength to eater remaining in liquid form. It takes additional
energy to change phases. There needs to be an addition of 540 calories per gram of heat
energy in order for liquid water to become water vapor. There is an 80 calorie per gram
difference between ice and liquid water.
Water's high heat capacity is a fundamental part of it remaining in liquid form as well. It
takes a relatively large amount of energy to raise the temperature of one gram of water
by 1 Celsius.
Answer to Question 2ANS:Answer
should include:
Hydrogen bonds form in water when the hydrogen atom of one water molecule is
attracted to the oxygen atom of another molecule. This is due to water being a polar
molecule which creates partially positive and negative portions of each molecule.
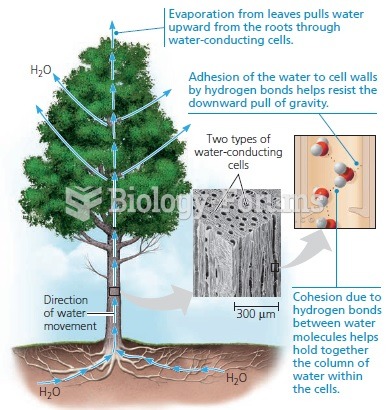
Hydrogen bonds allow water molecules to link together. This allows water molecules to
stick to one another, a property called cohesion. Cohesion leads to high surface tension
for water. Hydrogen bonds also influence waters adhesion to solids as well. Both
cohesion and adhesion cause capillary actions to occur. These properties contribute to
water remaining a liquid at normal temperatures and pressures.
Hydrogen bonds also are responsible for the color of water. The vibrations of these
bonds absorb red light energy while reflecting and scattering blue light.
The phase of water has a large influence on the hydrogen bonds. When water is a solid,
the hydrogen bonds become compressed and very rigid as the water molecules begin to
stack. They eventually form in hexagonal patterns. The hydrogen bonds are unable to
hold when water transitions from liquid to a gas.