Answer to Question 1Water temperature rises as the suns energy is absorbed and changed to heat, but water has a
very high heat capacity, so its temperature will not rise very much even if a large quantity of
heat is added. This tendency of a substance to resist a change in temperature with the gain or loss of heat energy is called thermal inertia. An example of the ocean's thermal inertia
compared to land's thermal inertia is two cities in the U.S.: San Francisco, California, and
Norfolk, Virginia. These two cities are on the same line of latitude, each is the same distance
from the equator, yet San Francisco is warmer in the winter and cooler in the summer than
Norfolk. Wind tends to flow from west to east at this latitude. Thus, air in San Francisco has
moved over the ocean while air in Norfolk has approached over land. Water doesnt warm as
much as land in the summer, nor cool as much in winter - a demonstration of thermal inertia
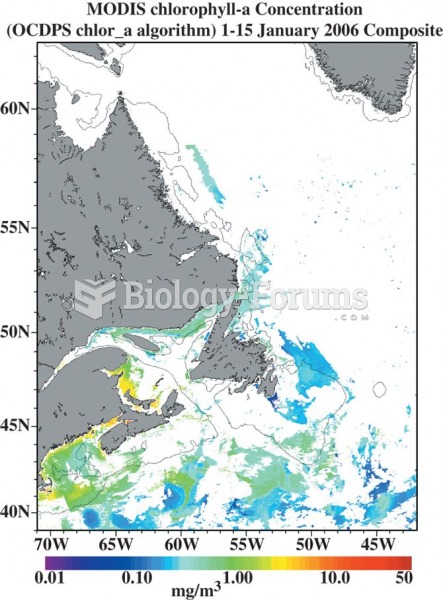
Answer to Question 2Seawater is about 96.5 pure water and 3.5 dissolved solids and gases. The solids
dissolved in seawater change its thermal characteristics, lowering its latent heat by about 4.
Only 0.96 calorie of heat energy is needed to raise the temperature of 1 gram of seawater by
1C. The dissolved solids also interfere with the formation of the ice lattice, acting as
antifreeze to lower the freezing point. The saltier the water, the lower the freezing point.
Seawaters density simply increases smoothly with decreasing temperature until it freezes.
The crystals that form are pure water ice, with the seawater salts excluded. The leftover cold,
salty water is very dense. Some of this water may be trapped among the ice crystals, but most
is free to fall toward the seabed, pulled rapidly downward by its great density. Seawater
evaporates more slowly than freshwater under identical circumstances because the dissolved
salts tend to attract and hold water molecules. The latent heat of evaporation, however, is
essentially the same for both freshwater and seawater. Salts are left behind as seawater
evaporates.