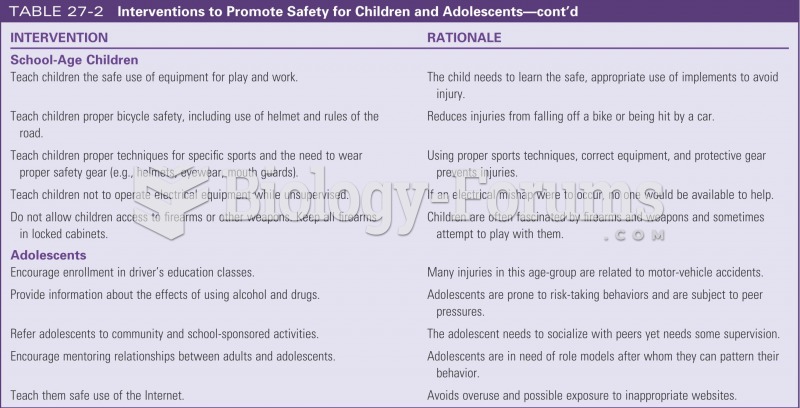
Give reasons for the following safety rules.
(a) Contact lenses should not be worn in the laboratory.
(b) A chemical spill on the skin should be washed off with water, not with solvent.
(c) Solvents are not to be poured down the sink.
(d) Water should not be used to extinguish laboratory fires.
(e) To dilute concentrated sulfuric acid, we pour it onto ice instead of simply mixing it with
water.
(f) Broken glassware should be picked up immediately and put in the designated container.
(g) Closed-toe shoes should be worn to laboratory.
Question 2Consider the following reaction:

a)
Predict the major product of the reaction.
b)
If steps 2 and 3 were reversed, what effect would this have on the major product?
Question 3Predict the major product of the following reaction:

Question 4Instructions: Consider the reaction below to answer the following question(s).

Refer to instructions. Draw the structure of product D.
Question 5Instructions: Consider the reaction below to answer the following question(s).

Refer to instructions. This reaction proceeds _____________ (faster or slower) than benzene.
Question 6Instructions: Consider the reaction below to answer the following question(s).

Refer to instructions. The Lewis acid catalyst in the reaction is indicated by letter _____.
Question 7Instructions: Consider the reaction below to answer the following question(s).

Refer to instructions. The nucleophile in the reaction is indicated by letter _____.
Question 8Consider the following compound:

a)
Provide the correct IUPAC name for the compound.
b)
Draw and name the other isomeric nitrotoluenes.
Question 9Draw the major product of the following reaction:

Question 10Place the following in order of reactivity towards electrophilic aromatic substitution.

a.
I > II > III > IV
b.
I > II > IV > III
c.
II > I > III > IV
d.
II > I > IV > III