This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
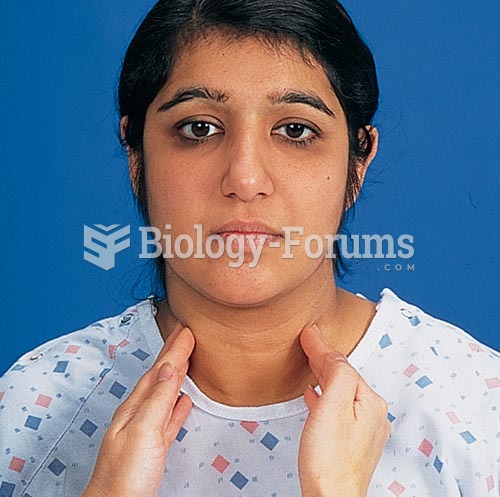
Multiple experimental evidences have confirmed that at the molecular level, cancer is caused by lesions in cellular DNA.
Did you know?
Fewer than 10% of babies are born on their exact due dates, 50% are born within 1 week of the due date, and 90% are born within 2 weeks of the date.
Did you know?
Women are 50% to 75% more likely than men to experience an adverse drug reaction.
Did you know?
Nearly 31 million adults in America have a total cholesterol level that is more than 240 mg per dL.
Did you know?
The human body's pharmacokinetics are quite varied. Our hair holds onto drugs longer than our urine, blood, or saliva. For example, alcohol can be detected in the hair for up to 90 days after it was consumed. The same is true for marijuana, cocaine, ecstasy, heroin, methamphetamine, and nicotine.
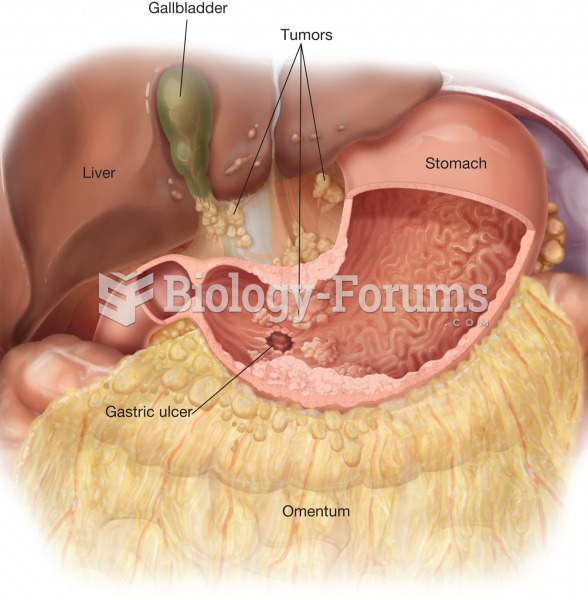 Gastric cancer. In advanced stages of gastric cancer, malignant cells spread to form tumors in the l
Gastric cancer. In advanced stages of gastric cancer, malignant cells spread to form tumors in the l
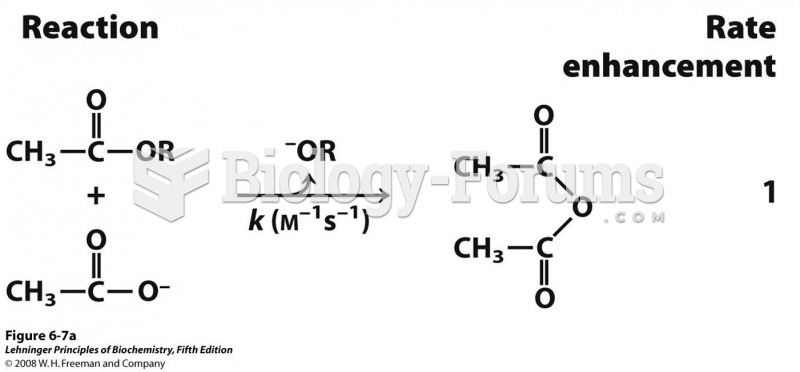 reactions of an ester with a carboxylate group to form an anhydride. The R group is the same in each
reactions of an ester with a carboxylate group to form an anhydride. The R group is the same in each