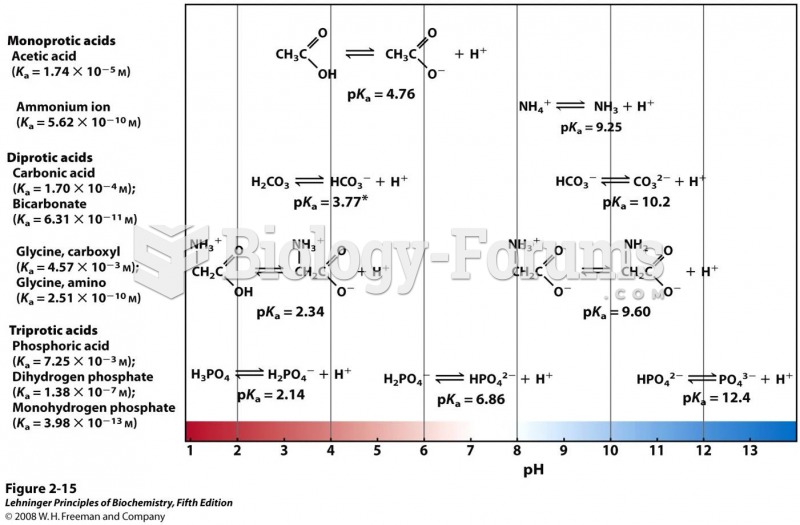
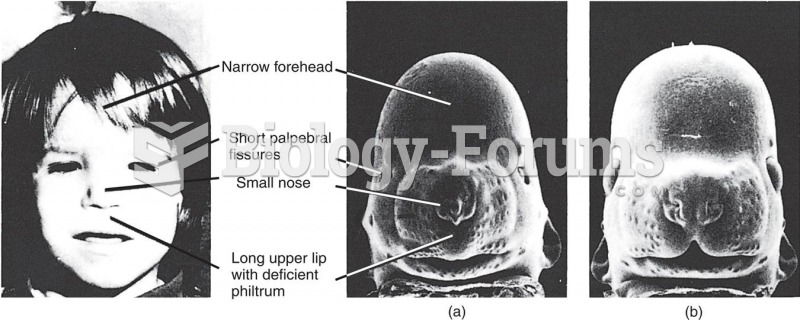
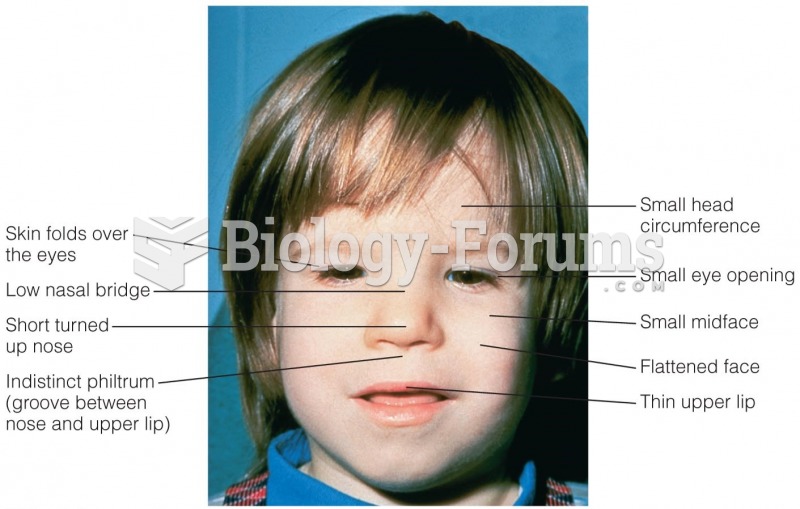
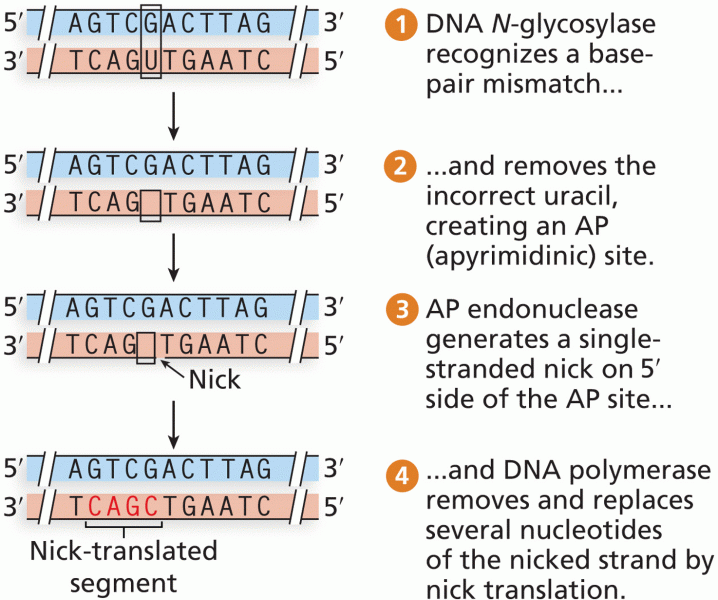
|
|
|
Amoebae are the simplest type of protozoans, and are characterized by a feeding and dividing trophozoite stage that moves by temporary extensions called pseudopodia or false feet.
The liver is the only organ that has the ability to regenerate itself after certain types of damage. As much as 25% of the liver can be removed, and it will still regenerate back to its original shape and size. However, the liver cannot regenerate after severe damage caused by alcohol.
You should not take more than 1,000 mg of vitamin E per day. Doses above this amount increase the risk of bleeding problems that can lead to a stroke.
There are approximately 3 million unintended pregnancies in the United States each year.
In 1886, William Bates reported on the discovery of a substance produced by the adrenal gland that turned out to be epinephrine (adrenaline). In 1904, this drug was first artificially synthesized by Friedrich Stolz.