Answer to Question 1 Answer to Question 2
Answer to Question 23-Cyclopentanediol has three stereoisomers. The two trans isomers are enantiomers
Answer to Question 31,4-Cyclohexanediol can exist as a pair of cis, trans isomers. Each is achiral because of a plane of symmetry
that bisects each molecule into two mirror halves. In the figure below, the plane of symmetry in each molecule
is in the plane of the paper. Because each isomer is achiral, there are only two stereoisomers of 1,4-
cyclohexanediol.
 Answer to Question 4
Answer to Question 4(a)
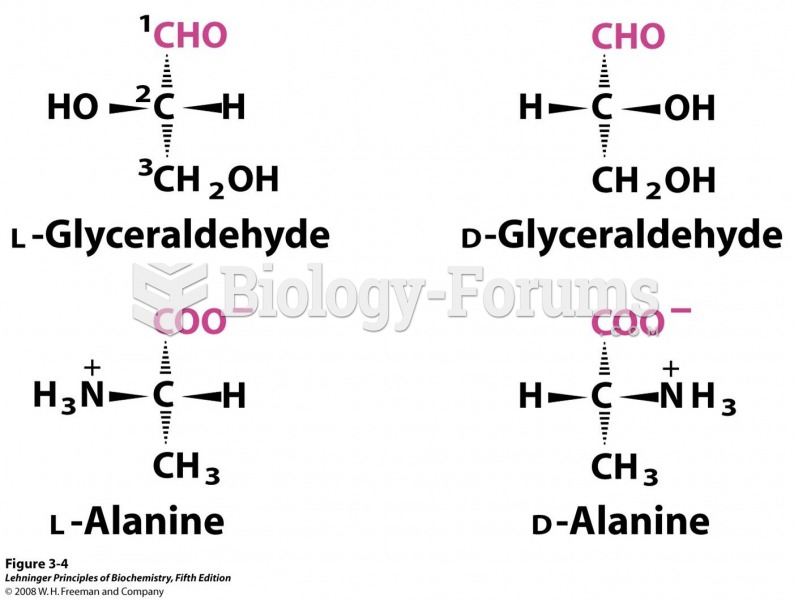
Compounds (3) and (4) are the same compound having the configuration (2S,3S). Compound (2) has the
configuration of (2R,3S) while compound (1) is (2R,3R).
(b)
Compounds (3) and (1) or (4) and (1) represent pairs of enantiomers (recall that (3) and (4) are the same).
(c)
The meso compound of tartaric acid has the (2R,3S) configuration so compound (2) is the meso compound.
(d)
The best way to analyze this is in terms of the various possible pairs of diastereomers. Compounds (3)/(4) and
(2) are diastereomers. Compounds (1) and (2) are also a pair of diastereomers
Answer to Question 5Answer to Question 6(a)
The configuration of each chiral center is labeled on the above structures. This labeling often helps when
trying to establish stereochemical relationships among molecules.
(b)
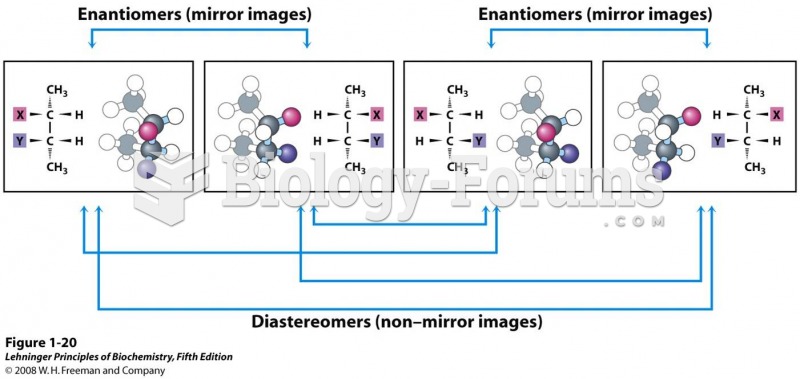
Enantiomers are stereoisomers that are mirror images of each other. The pairs of enantiomers are structures
(1) and (3) (S,S and R,R) as well as structures (2) and (4) (S,R and R,S).
(c)
Diastereomers are stereoisomers that are not mirror images of each other. Therefore, there are several sets of
diastereomers. The following chart describes the relationship between any pair of molecules
![]()