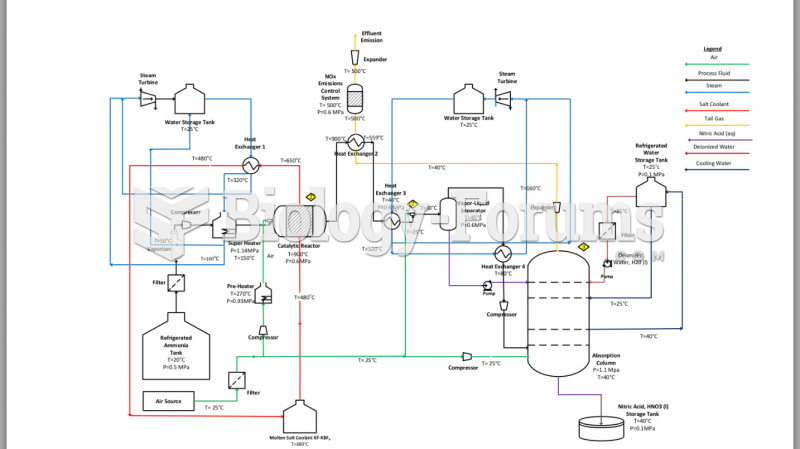
Using cartoon representations, draw a molecular orbital mixing diagram for a C-O -bond. In your picture, consider the relative energies of C and O, and how this changes the resulting bonding and antibonding molecular orbitals relative to a C-C -bond.
 Question 2
Question 2Ic1zlOLlNkQVp5kQptI46QdCIHyqJ9hCqbY9oHhhAhdh32ynr44YfDGhLK4Dr5ogRB/lRx4Bl1aDakg9CYuuzOEagJgYpPUE2p/bojkCYCTz75ZFjFjcDDIeQk3CQcEXo9evSwu+++O2xrzoI5puUyVVW2AfLyxg3hEMfbePQNHcEtgS7hrHrIi5BmthV1KS3xCGwEJuXRHq4hoJWXkHjajccpnYiCOJWpOEK1QyF1oeEoJJ5jedrIMfHURYinzYpTXYTYi1h8iOM6eJA2E8ewIG2DqCF7d45ATQg4edSEkF+vEwJsAfLiiy8GImAdRlS4IZgRlo8//njY6+mxxx4LxMBbNQZ1BDqCHaHPcBMzkXhDhkAgI0hEBIJAx0cFMGUVDZv3AAALCUlEQVQjECWIISOmvxIPEeHRbrCzQBgIYOqkjbRNocqmHI4JIQsJdOJ0TKhrHOucdtEWkQf9Io6QOELS0ia0LbVP6VUWeTjGMczUoUMHO/LIIwMWdblRbEFPnUxjducI1AYBJ4/aoORpMkIAocpwFB9KgkQwhDPUQjwzh1iwx6wqDOcYfxGGpBs6dGhYFIfgZA8q1ilAPBiJEW4MqyDwIQ8JfjWQsiW8EYYQEWSBwCcvZXIdAc01PEQkIoOM9AZPXNSJPFQ+54pDoFMPIXGkwSuOeJGFiEN5OKetpOdY5ME5eSA44vGURxyGbexAzJJik8VzzjknzJ6ivdQvF+0D2HBOiFMIcVLXpptuGvBUXg8dgeoQcPKoDh2/VicEEGIYwZlKyoZ/xx9/fKXymGXFQrXoNFz2eEIwQizs+8Q2HQhQ4iAAhmq0XgMtRENZCEGchDbCVm/uCGdIB8FMnAQ58Wg2tFXajDSOqKAlTkKZ8hH8CF88ZSHUiccTR1oJfNLKq27Siwhog0iN42g+2kvfITnK5jqkeeKJJ4b1G6zjgDh69eoVZrUxpVlTfWmH+kAb6YOG5GgfHk2Lz+2yUJA2cu7OEagNAk4etUHJ02SEAIILobX99tsbtg8WuGHXQPAfcMABYaU46w1wCDIJO4Qcs4nYl4rNDllhzlYkzJZCMKKB4BnGksBH6Ik8KA9BKIJA+FI+9SKMEc6EGOARxhqqQjiTB+EsAqFNapfayLnSUo8Ig3jyk454yoYgdK72iNC4xrHS6brIQ9dEeKSjLLQ5Fv7hIBF247366quDBoIB/bDDDgubKjIDC2LlPtCnqAMr4YX2As58CdHJI4qSH1eHgJNHdej4tTohgNBCQCHwEPoMSeGjToJYwpoQYQw5PPTQQ3bfffeFldBM6YVMIAC2UWd9AoZddqdFQCL0EJB46qRcCXaEMW0gDSGaBhoMb/MYiJUeYc2QFemithTaqzd4EQXlIOypByeyIpTnOgRBGhGFQq5RnwhCx1wXeZAG4qCdEAeOob+///3v4Zjr4AWpXnHFFTZo0CC79957bdKkSWFdDeTKUBRrP5i9xrfWwZ/y58yZE8iTVeksxGQdzjHHHBPwoa/uHIGaEHDyqAkhv54xAgihmgQR1xHUUac3YkjilFNOCZ79nfi069SpU8PaEHbMxfgNiUAEkBOaBIIfTxkIV4Q8Ah+HgEaQY28hL0RDWtqAcI6SEPkRzDhC8uEpT55yOYYsEPp44jhXSBs4V6h0IokoaXCMYCekPXhpTWhvrAZntb0cbadNcuBVUlISPNuPsBHkf//730C67Dq8YMECmzJlSsCL2VW0HczYeJGFmDj1WWV66AhUhUDFf21VqTzeEcgxAgxv4U877bTw1nzPPfcYHhKRi87GghgQhHiEJMIaYYxgRvNgGEwkRX7IBMENkeHJp1ACWuRBCDkQQgyUn0wWxIs0RBSEpMNHiUKEQZzi0TYon3ZgE2IlOTYb4qKEHD3mGo447ER4vgkSddQd7Xf0mh87Aukg4OSRDlqeNicIIJwRjHgEOquq8WeffbY999xzQRvhWx1oJ4sXL66xjZAHw2KE0jaog2MNexFSlxwCWSQhAuCceNpFHOQgL02DEB8lDshCRCayUBzDVDgE/K677hqM4diHcNQBmVTnRCBKw7kIhjBKHLRfrqZylc5DR0AIrPx3KMZDR6CBESgb2cVaD5odau1cutDG2RU2tfdoK2m1vCEIPQlAYiQgeRNnoRwex86z7CT7+uuvBwMwRmAErvIzHRhBzqaBaB6UA2HgiBeRiEAQqOSVAFa9hCIQjkUQCqV1IJw5hjggCfKQBqIgjiEpjlnDorL5njpbtTB7CoO3hDrX1Z4oXmadrXThrApYhQ5F7DTqA2XghaVwUd3K56EjUBsEnDxqg5KnqTcEpg9sZL3mltrC8lkGVywXjGalvVdWKWGnmORzxWP/wCcb5XWdEAHORoAsXJQ2gABnRXt05hb2BDSPaF0cizQoC6FLGSILrkk7EWlwPZoGEoE0MNSTHsd0ZgiQWVLs7SVCCxdX/KgdyXhZ2Ujr0rqRzZtWbqN7VjZ0i3yUX2GqsqNxfuwI1ISAk0dNCPn1+kOgbKSNGMObc0kgDipqVTLLytsMtIELOUm/ar1FpxKSCHQI4aabbgq70GJURtgj3NFiENpcxzO8A4HIoE5LKFPkQT556oQUotqF0hHHEBVpOYaocNgj9tprr2CTQCNiEWSNLgVe1qrExpVOsNYjRtoZPVfiWGNZnsARqCMCTh51BNCzZ45A2dQJNrvzYTYumSR6jrbRGRSLEEdIpyIOipPwx14yefLksD4CAmEoCW1Amgchb+wQCGVGy+MYL5KShkEZxEEaxHEOaWD4jrrf//734fsZrM1IJgzarnZG61T+qvBq1fsw6zxogk0tK0kMXymPh45AfSHg5FFfyHq5DY4AAjdqEE5uQPQa3xVh+IqpwGyTgqDHsW6E6asiD2khIgyRCSGaBCSB0Ed7kRFc9VIGW6uwxoJFffvtt1+wZ1BmKqchplTXPM4RiBsCqZ/iuLXS2+MIZBkBBD7GaVa+s3bkgQceCOtH2CCQFe3JDvLAI+DJKy1B6TCyQxJ8KbF9+/a27bbbhqnFbdu2DesqosRFXpWn/B46AvmGgJNHvt2xAmpvlcMtZSNt5MISK+lZf51Fc0CIQwZ8eArPkBOr2PkGCcNZTJvFsP3JJ5+EGVxchwQYlsKxNgR7BUNRLOJDu2CjxlSOuqgTR52QR7quKryqGs5Kt3xP7wikg4CTRzpoedrsItCqxM4fMMh69R9pvWfJ2DvdBvY3O2NWdqtKLg0SQKBDCBLmxKGN4JMdmzIyNKV85GWrFAgkleO6nIiCenA61/Vah6nwKhtp/QfNtgHTls9Wq3VZntARqCMCTh51BNCz1w2BnqPLbdrARta60aAVBQ2waeWjM5lolXZDJMxrk1EfXapNWtIkl50xYSRVCF4LWReTwIvZauVuKE/CyU/rHwEnj/rH2GuoAQEEYnkm06tqKLdQL4fpzCWF2jvvV74gUP1eB/nSC2+nI+AIOAKOQIMi4OTRoHB7ZY6AI+AIFAYCTh6FcR+9F46AI+AINCgCbvNoULi9MkfAEagtAqWlpSmT8tErd7lHwMkj9/fAW+AIOAJVIJBMFFURShXZPboeEfBhq3oE14t2BBwBR6BQEXDyKNQ76/1yBBwBR6AeEXDyqEdwvWhHwBFwBAoVAbd5FOqd9X45AgWAgNs44nsTnTzie2+8ZY5AUSOQbCwvajBi2HkftorhTfEmOQKOgCMQdwScPOJ+h7x9joAj4AjEEAEnjxjeFG+SI+AIOAJxR8DJI+53yNvnCDgCjkAMEXDyiOFN8SY5Ao6AIxB3BJw84n6HvH2OgCPgCMQQASePGN4Ub5Ij4Ag4AnFHwMkj7nfI2+cIOAKOQAwRqJI8ysvLLVvfXY5hv71JjoAj4Ag4AnVAoEry+M1vfmMQCE5hcj2//vprcpSfOwKOgCPgCBQBAlWSB30XOUQ1kCipcOzOEXAEHAFHoPgQqFL6//jjj/bLL79UQgTCaNy4caV4j3AEHAFHwBEoHgQalVcxJvXOO+/Yt99+a82bN7eSkhL77rvvbJVVVrG5c+dakyZNbOutt7aff/65eJDynjoCjoAj4AgkEKiSPBIp/MARcAQcAUfAEUhCoMphq6R0fuoIOAKOgCPgCCQQcPJIQOEHjoAj4Ag4ArVFwMmjtkh5OkfAEXAEHIEEAv8PQvPod3vXucwAAAAASUVORK5CYII= />"
Question 3What is the hybridization of the highlighted atoms in the following structures, and what are your estimates for the bond angles around these highlighted atoms? In each case, in what kind of orbital does the lone pair of electrons on the nitrogen reside.
Question 4Draw structural formulas for
(a) The four primary (1) amines with the molecular formula C
4H
1 1N.
(b) The three secondary (2) amines with the molecular formula C
4H
1 1N.
(c) The one tertiary (3) amine with the molecular formula C
4H
1 1N.
Question 5What is the meaning of the term tertiary (3) when it is used to classify amines? Draw a structural formula for the one tertiary (3) amine known as Hunigs base (
N,N-diisopropylethylamine).
Question 6What is the meaning of the term tertiary (3) when it is used to classify alcohols? Draw a structural formula for the one tertiary (3) alcohol with the molecular formula C
4 H
1 0O.
Question 7Draw Lewis structures for these functional groups. Be certain to show all valence electrons on each.
(a) Carbonyl group
(b) Carboxyl group
(c) Hydroxyl group
(d) Ester group
(e) Amide group
Question 8Use VSEPR to predict bond angles about each highlighted atom.