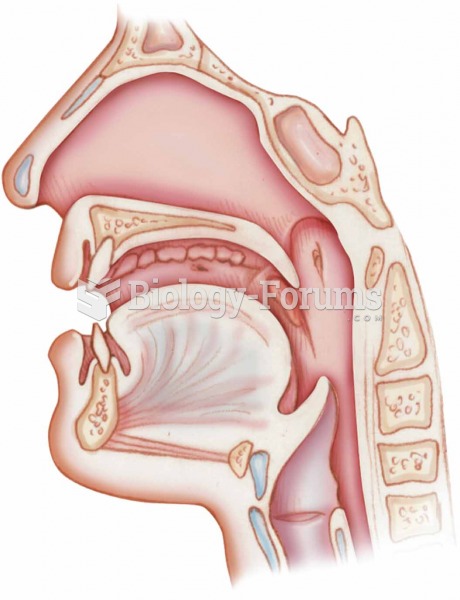
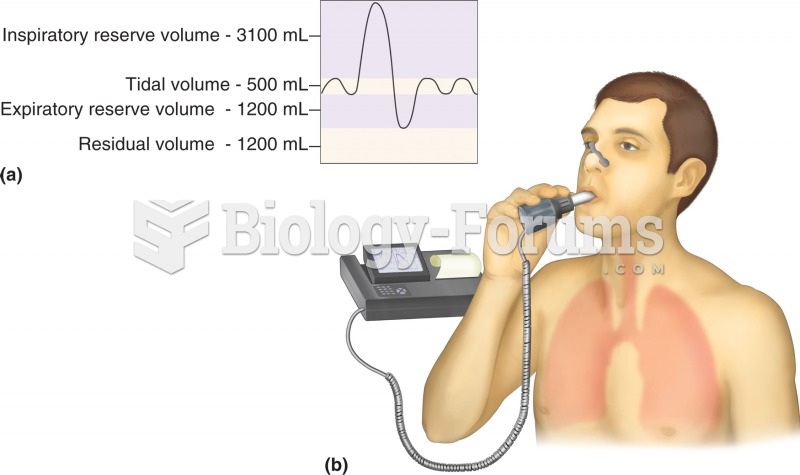
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Serum cholesterol testing in adults is recommended every 1 to 5 years. People with diabetes and a family history of high cholesterol should be tested even more frequently.
Did you know?
After 5 years of being diagnosed with rheumatoid arthritis, one every three patients will no longer be able to work.
Did you know?
The term bacteria was devised in the 19th century by German biologist Ferdinand Cohn. He based it on the Greek word "bakterion" meaning a small rod or staff. Cohn is considered to be the father of modern bacteriology.
Did you know?
The first documented use of surgical anesthesia in the United States was in Connecticut in 1844.
Did you know?
By definition, when a medication is administered intravenously, its bioavailability is 100%.