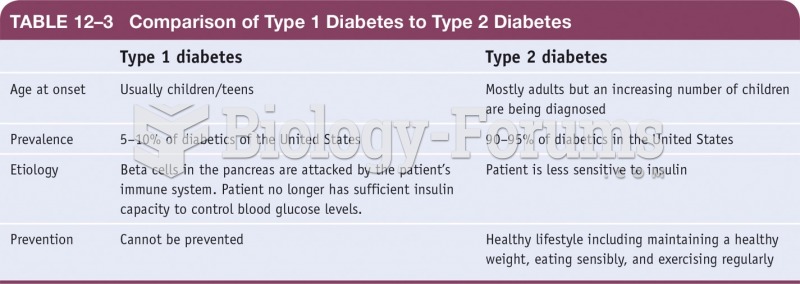
|
|
|
Though newer “smart” infusion pumps are increasingly becoming more sophisticated, they cannot prevent all programming and administration errors. Health care professionals that use smart infusion pumps must still practice the rights of medication administration and have other professionals double-check all high-risk infusions.
Thyroid conditions may make getting pregnant impossible.
Approximately 500,000 babies are born each year in the United States to teenage mothers.
In 1885, the Lloyd Manufacturing Company of Albany, New York, promoted and sold "Cocaine Toothache Drops" at 15 cents per bottle! In 1914, the Harrison Narcotic Act brought the sale and distribution of this drug under federal control.
Cancer has been around as long as humankind, but only in the second half of the twentieth century did the number of cancer cases explode.