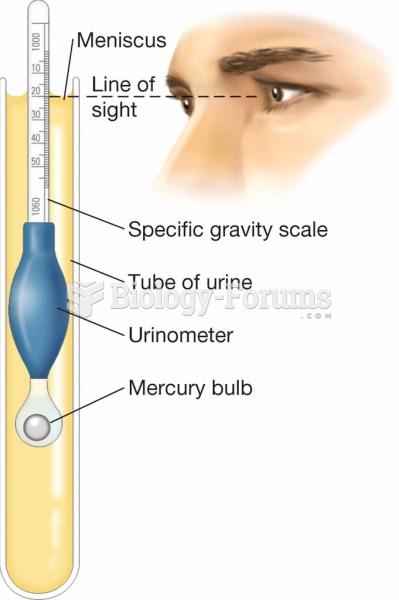
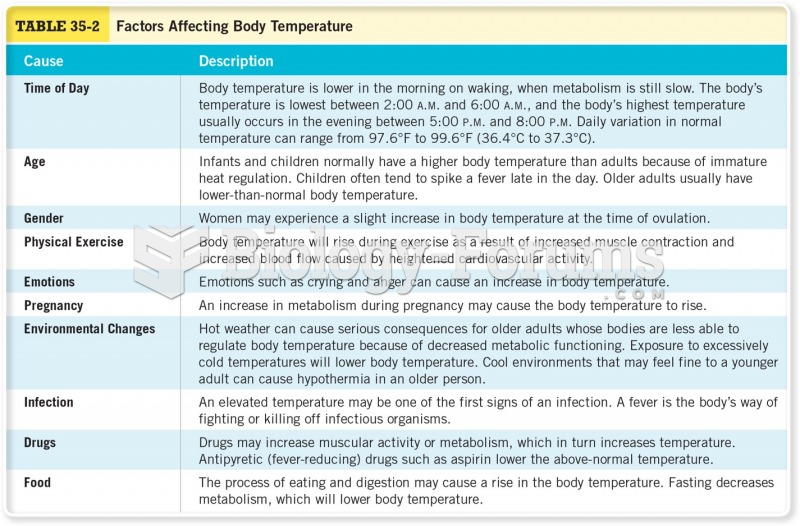
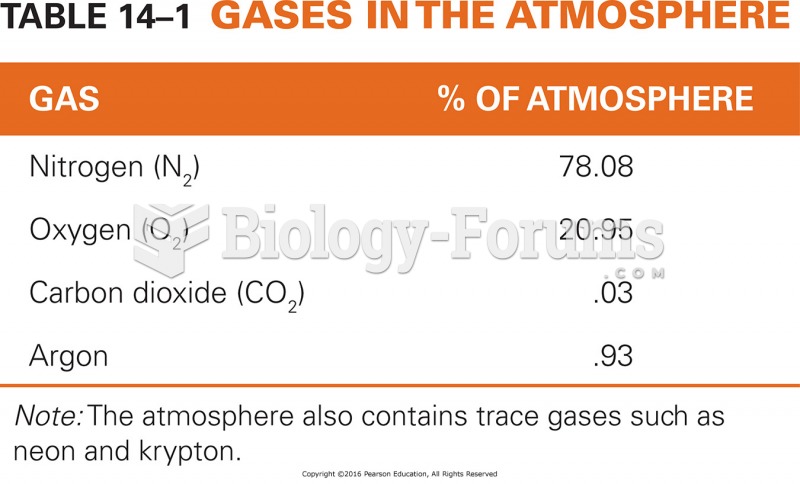
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Common abbreviations that cause medication errors include U (unit), mg (milligram), QD (every day), SC (subcutaneous), TIW (three times per week), D/C (discharge or discontinue), HS (at bedtime or "hours of sleep"), cc (cubic centimeters), and AU (each ear).
Did you know?
Between 1999 and 2012, American adults with high total cholesterol decreased from 18.3% to 12.9%
Did you know?
Pubic lice (crabs) are usually spread through sexual contact. You cannot catch them by using a public toilet.
Did you know?
In ancient Rome, many of the richer people in the population had lead-induced gout. The reason for this is unclear. Lead poisoning has also been linked to madness.
Did you know?
Cytomegalovirus affects nearly the same amount of newborns every year as Down syndrome.