This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
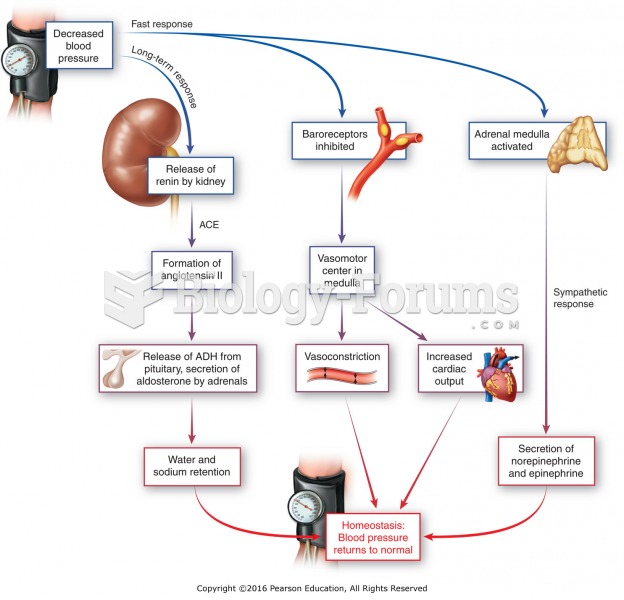
Your chance of developing a kidney stone is 1 in 10. In recent years, approximately 3.7 million people in the United States were diagnosed with a kidney disease.
Did you know?
A seasonal flu vaccine is the best way to reduce the chances you will get seasonal influenza and spread it to others.
Did you know?
Your heart beats over 36 million times a year.
Did you know?
Congestive heart failure is a serious disorder that carries a reduced life expectancy. Heart failure is usually a chronic illness, and it may worsen with infection or other physical stressors.
Did you know?
Medication errors are more common among seriously ill patients than with those with minor conditions.