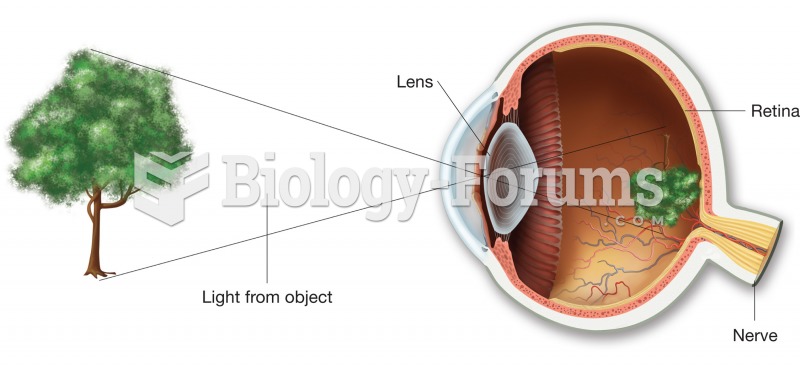
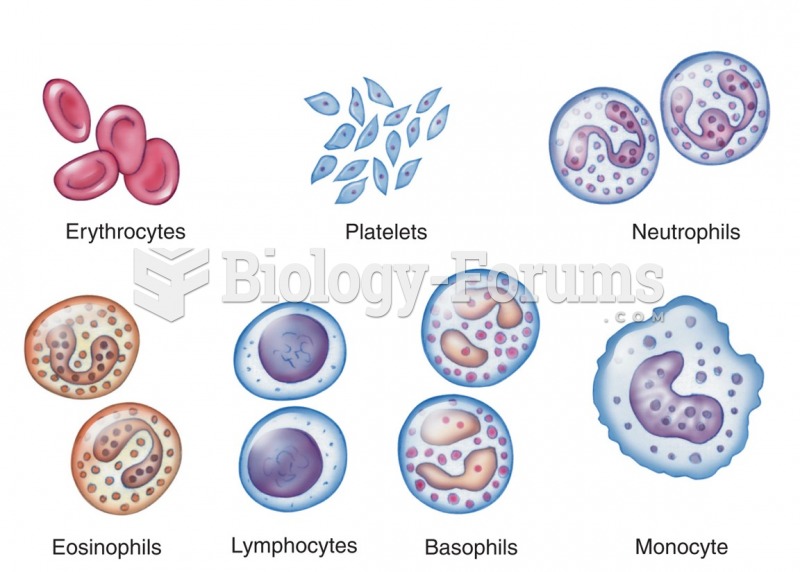
|
|
|
The first successful kidney transplant was performed in 1954 and occurred in Boston. A kidney from an identical twin was transplanted into his dying brother's body and was not rejected because it did not appear foreign to his body.
The ratio of hydrogen atoms to oxygen in water (H2O) is 2:1.
Illicit drug use costs the United States approximately $181 billion every year.
About 80% of major fungal systemic infections are due to Candida albicans. Another form, Candida peritonitis, occurs most often in postoperative patients. A rare disease, Candida meningitis, may follow leukemia, kidney transplant, other immunosuppressed factors, or when suffering from Candida septicemia.
Multiple experimental evidences have confirmed that at the molecular level, cancer is caused by lesions in cellular DNA.