|
|
|
More than one-third of adult Americans are obese. Diseases that kill the largest number of people annually, such as heart disease, cancer, diabetes, stroke, and hypertension, can be attributed to diet.
Studies show that systolic blood pressure can be significantly lowered by taking statins. In fact, the higher the patient's baseline blood pressure, the greater the effect of statins on his or her blood pressure.
Looking at the sun may not only cause headache and distort your vision temporarily, but it can also cause permanent eye damage. Any exposure to sunlight adds to the cumulative effects of ultraviolet (UV) radiation on your eyes. UV exposure has been linked to eye disorders such as macular degeneration, solar retinitis, and corneal dystrophies.
Asthma is the most common chronic childhood disease in the world. Most children who develop asthma have symptoms before they are 5 years old.
Between 1999 and 2012, American adults with high total cholesterol decreased from 18.3% to 12.9%
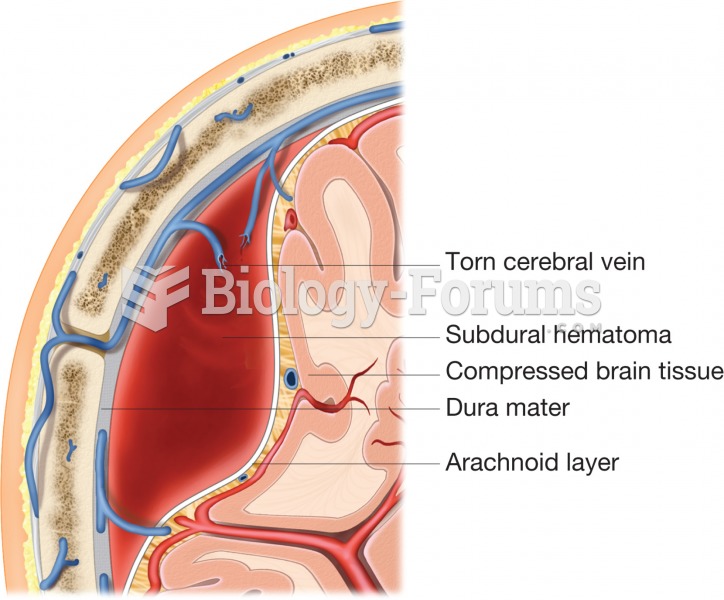 A subdural hematoma. A meningeal vein is ruptured and blood has accumulated in the subdural space, p
A subdural hematoma. A meningeal vein is ruptured and blood has accumulated in the subdural space, p
 Block Diagram of a Dual-stage Pressure System for Dual-Stage Pressure Production of Nitric Acid Seme
Block Diagram of a Dual-stage Pressure System for Dual-Stage Pressure Production of Nitric Acid Seme
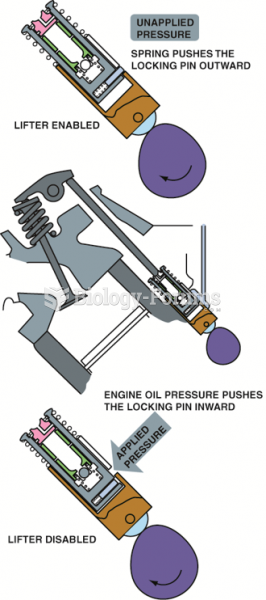
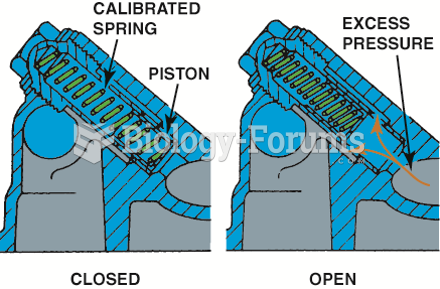 Oil pressure relief valves are spring loaded. The stronger the spring tension, the higher the oil ...
Oil pressure relief valves are spring loaded. The stronger the spring tension, the higher the oil ...