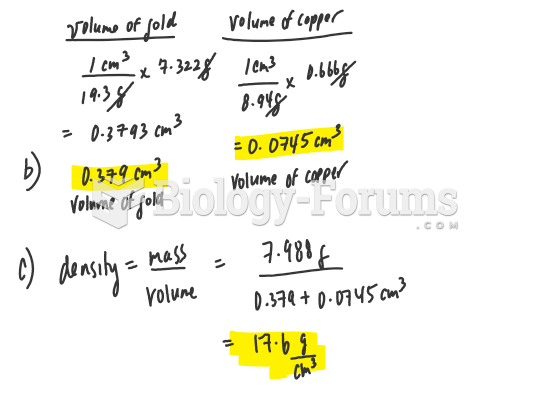
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Human kidneys will clean about 1 million gallons of blood in an average lifetime.
Did you know?
HIV testing reach is still limited. An estimated 40% of people with HIV (more than 14 million) remain undiagnosed and do not know their infection status.
Did you know?
Patients who have undergone chemotherapy for the treatment of cancer often complain of a lack of mental focus; memory loss; and a general diminution in abilities such as multitasking, attention span, and general mental agility.
Did you know?
Russia has the highest death rate from cardiovascular disease followed by the Ukraine, Romania, Hungary, and Poland.
Did you know?
The U.S. Preventive Services Task Force recommends that all women age 65 years of age or older should be screened with bone densitometry.