This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
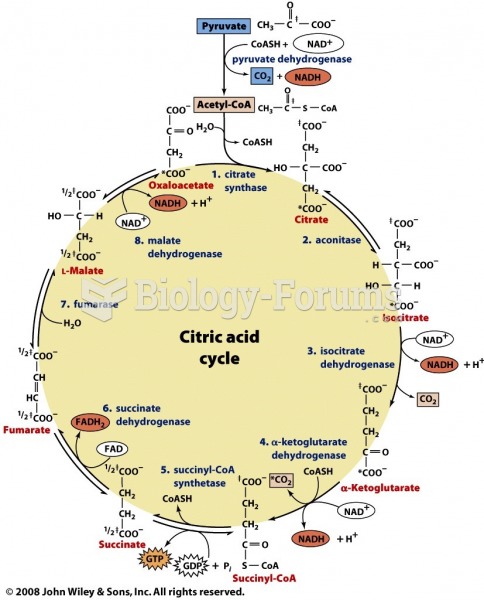
People with alcoholism are at a much greater risk of malnutrition than are other people and usually exhibit low levels of most vitamins (especially folic acid). This is because alcohol often takes the place of 50% of their daily intake of calories, with little nutritional value contained in it.
Did you know?
More than 4.4billion prescriptions were dispensed within the United States in 2016.
Did you know?
The immune system needs 9.5 hours of sleep in total darkness to recharge completely.
Did you know?
Thyroid conditions may make getting pregnant impossible.
Did you know?
Critical care patients are twice as likely to receive the wrong medication. Of these errors, 20% are life-threatening, and 42% require additional life-sustaining treatments.