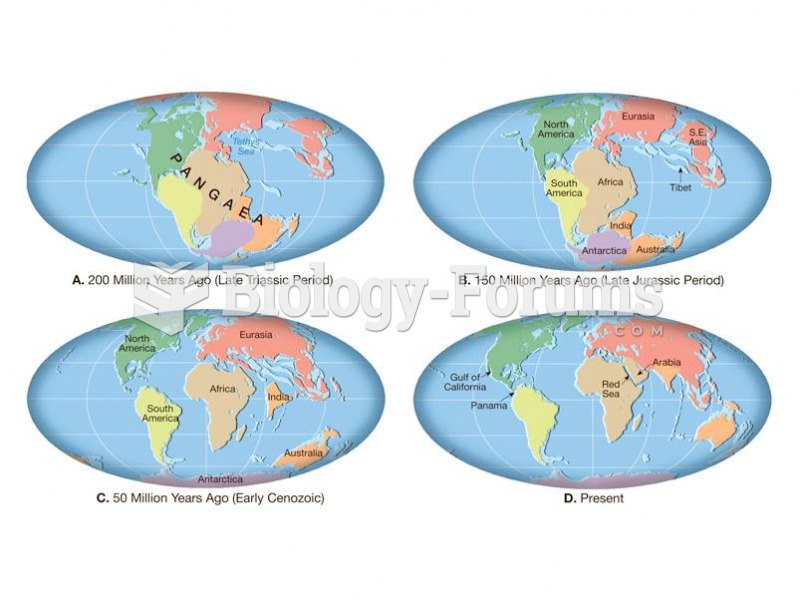
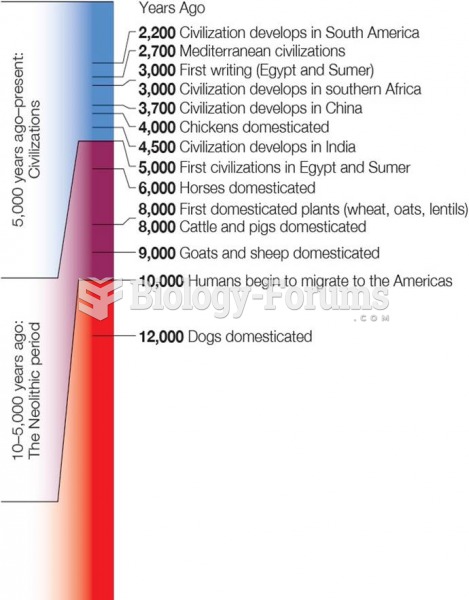
|
|
|
Asthma cases in Americans are about 75% higher today than they were in 1980.
More than 34,000 trademarked medication names and more than 10,000 generic medication names are in use in the United States.
In most climates, 8 to 10 glasses of water per day is recommended for adults. The best indicator for adequate fluid intake is frequent, clear urination.
Blood is approximately twice as thick as water because of the cells and other components found in it.
The top 10 most important tips that will help you grow old gracefully include (1) quit smoking, (2) keep your weight down, (3) take supplements, (4) skip a meal each day or fast 1 day per week, (5) get a pet, (6) get medical help for chronic pain, (7) walk regularly, (8) reduce arguments, (9) put live plants in your living space, and (10) do some weight training.