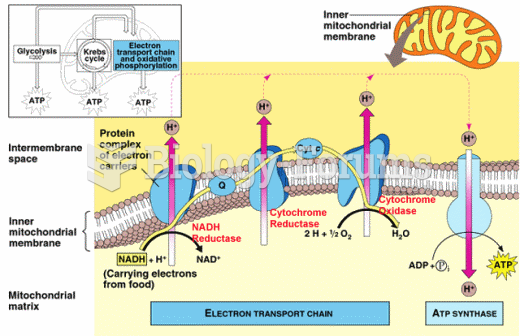
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
The average human gut is home to perhaps 500 to 1,000 different species of bacteria.
Did you know?
Russia has the highest death rate from cardiovascular disease followed by the Ukraine, Romania, Hungary, and Poland.
Did you know?
Vital signs (blood pressure, temperature, pulse rate, respiration rate) should be taken before any drug administration. Patients should be informed not to use tobacco or caffeine at least 30 minutes before their appointment.
Did you know?
On average, the stomach produces 2 L of hydrochloric acid per day.
Did you know?
The Romans did not use numerals to indicate fractions but instead used words to indicate parts of a whole.