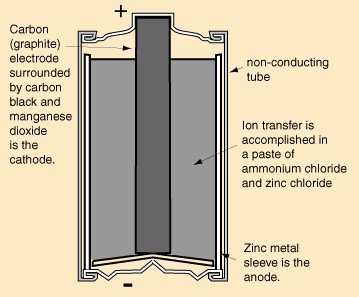
|
|
|
A headache when you wake up in the morning is indicative of sinusitis. Other symptoms of sinusitis can include fever, weakness, tiredness, a cough that may be more severe at night, and a runny nose or nasal congestion.
According to the Migraine Research Foundation, migraines are the third most prevalent illness in the world. Women are most affected (18%), followed by children of both sexes (10%), and men (6%).
The training of an anesthesiologist typically requires four years of college, 4 years of medical school, 1 year of internship, and 3 years of residency.
About 100 new prescription or over-the-counter drugs come into the U.S. market every year.
To prove that stomach ulcers were caused by bacteria and not by stress, a researcher consumed an entire laboratory beaker full of bacterial culture. After this, he did indeed develop stomach ulcers, and won the Nobel Prize for his discovery.
 Modern primate study sometimes involves high-tech methods. This golden lion tamarin is having a batt
Modern primate study sometimes involves high-tech methods. This golden lion tamarin is having a batt
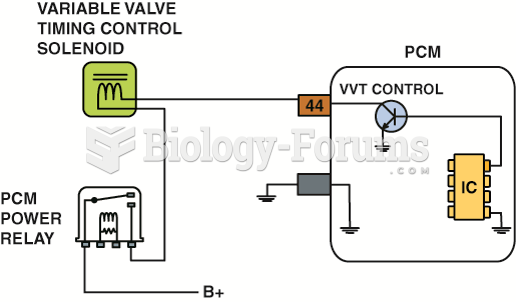 The schematic of a variable valve timing control circuit, showing that battery power (+) is being ...
The schematic of a variable valve timing control circuit, showing that battery power (+) is being ...
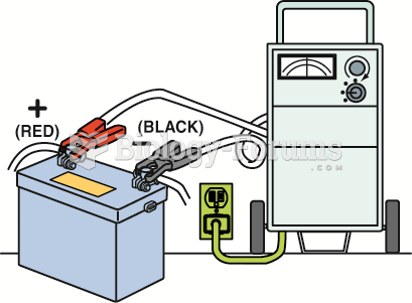 To use a battery charger, make sure the charger is connected to the battery before plugging in the ...
To use a battery charger, make sure the charger is connected to the battery before plugging in the ...