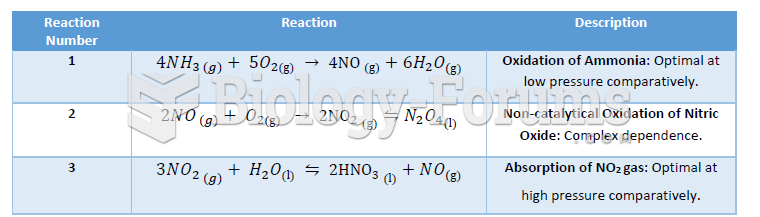
|
|
|
According to the FDA, adverse drug events harmed or killed approximately 1,200,000 people in the United States in the year 2015.
Prostaglandins were first isolated from human semen in Sweden in the 1930s. They were so named because the researcher thought that they came from the prostate gland. In fact, prostaglandins exist and are synthesized in almost every cell of the body.
The Romans did not use numerals to indicate fractions but instead used words to indicate parts of a whole.
In the United States, an estimated 50 million unnecessary antibiotics are prescribed for viral respiratory infections.
In 1885, the Lloyd Manufacturing Company of Albany, New York, promoted and sold "Cocaine Toothache Drops" at 15 cents per bottle! In 1914, the Harrison Narcotic Act brought the sale and distribution of this drug under federal control.
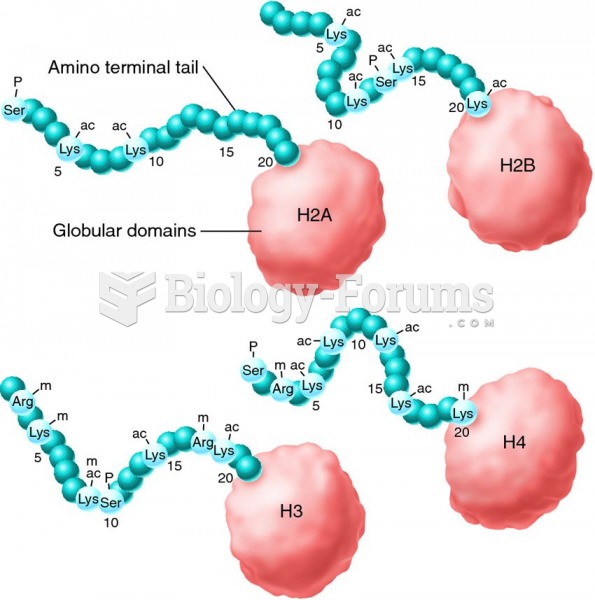 Examples of covalent modifications that occur to the amino terminal tails of histone proteins. The a
Examples of covalent modifications that occur to the amino terminal tails of histone proteins. The a
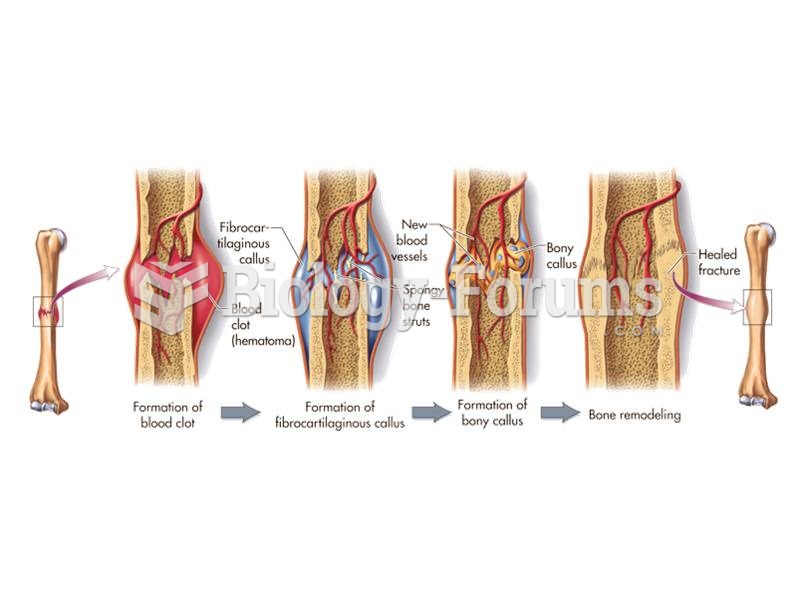 Bone fractures that occur before death show signs of healing. The process of fracture healing starts
Bone fractures that occur before death show signs of healing. The process of fracture healing starts