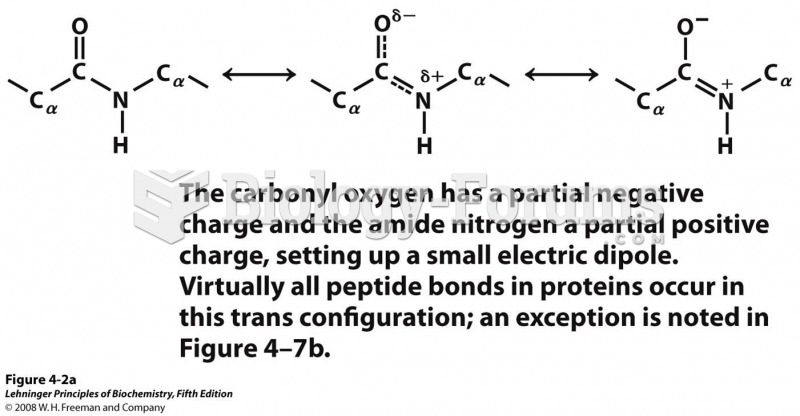
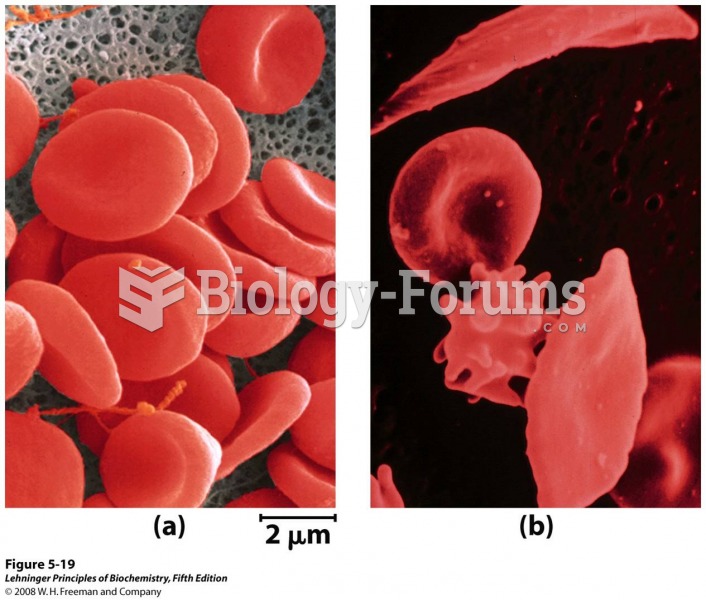
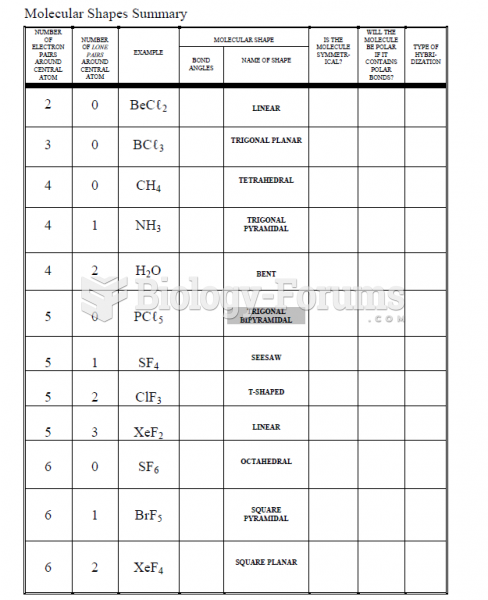
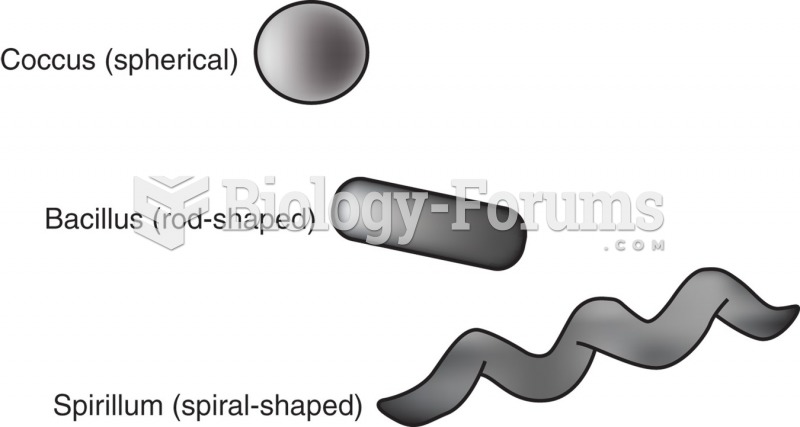
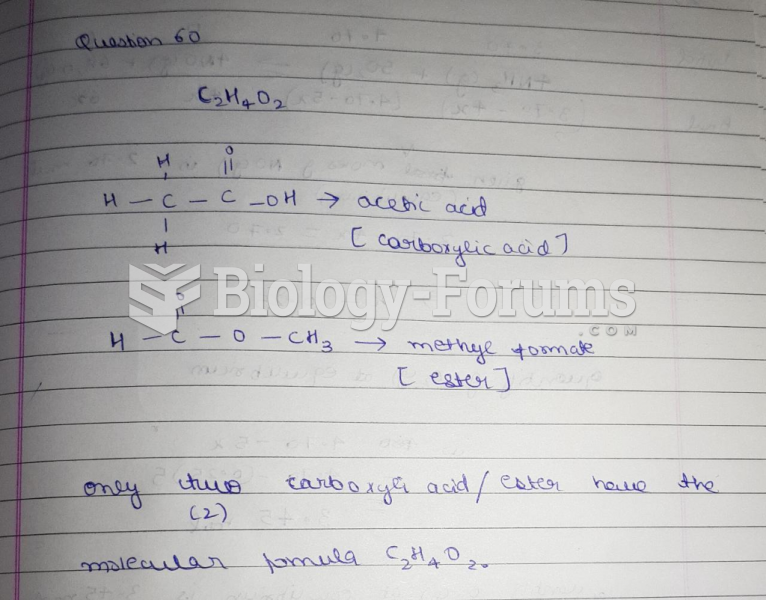
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Blastomycosis is often misdiagnosed, resulting in tragic outcomes. It is caused by a fungus living in moist soil, in wooded areas of the United States and Canada. If inhaled, the fungus can cause mild breathing problems that may worsen and cause serious illness and even death.
Did you know?
There are more bacteria in your mouth than there are people in the world.
Did you know?
Most childhood vaccines are 90–99% effective in preventing disease. Side effects are rarely serious.
Did you know?
People with high total cholesterol have about two times the risk for heart disease as people with ideal levels.
Did you know?
The heart is located in the center of the chest, with part of it tipped slightly so that it taps against the left side of the chest.