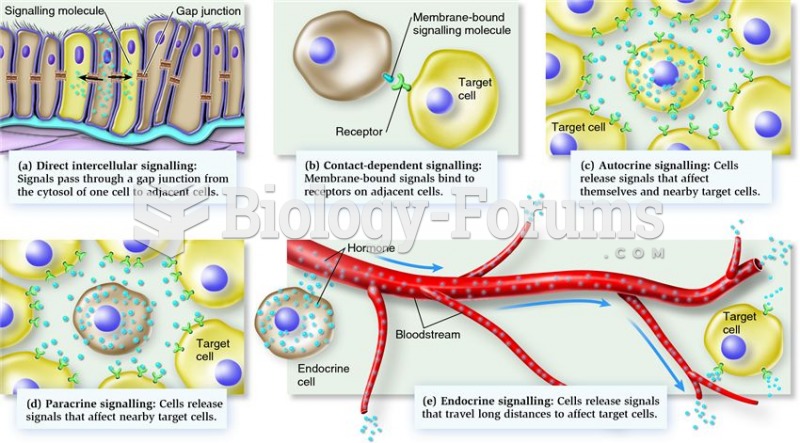
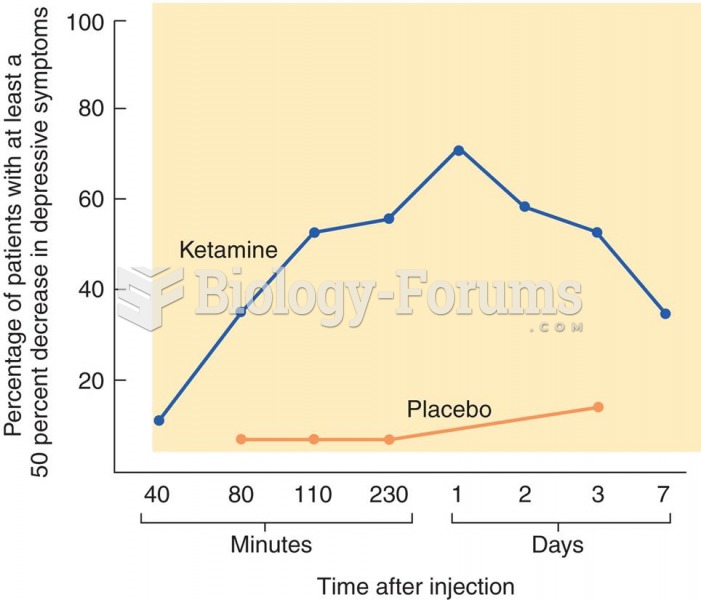
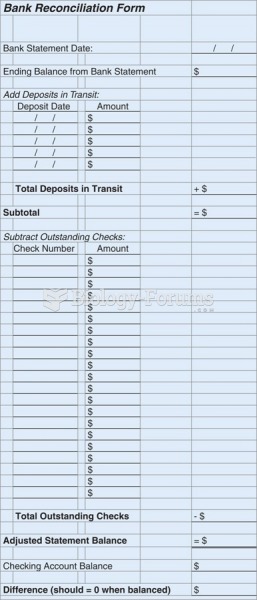
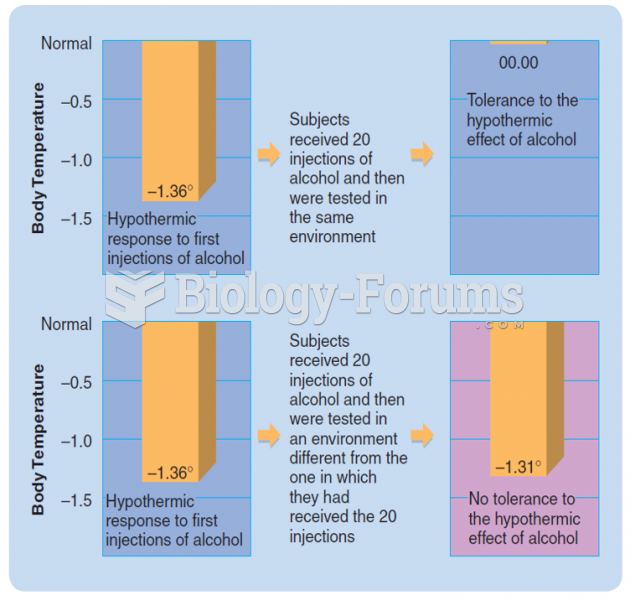
|
|
|
There are over 65,000 known species of protozoa. About 10,000 species are parasitic.
Serum cholesterol testing in adults is recommended every 1 to 5 years. People with diabetes and a family history of high cholesterol should be tested even more frequently.
Aspirin is the most widely used drug in the world. It has even been recognized as such by the Guinness Book of World Records.
In the United States, there is a birth every 8 seconds, according to the U.S. Census Bureau's Population Clock.
Once thought to have neurofibromatosis, Joseph Merrick (also known as "the elephant man") is now, in retrospect, thought by clinical experts to have had Proteus syndrome. This endocrine disease causes continued and abnormal growth of the bones, muscles, skin, and so on and can become completely debilitating with severe deformities occurring anywhere on the body.