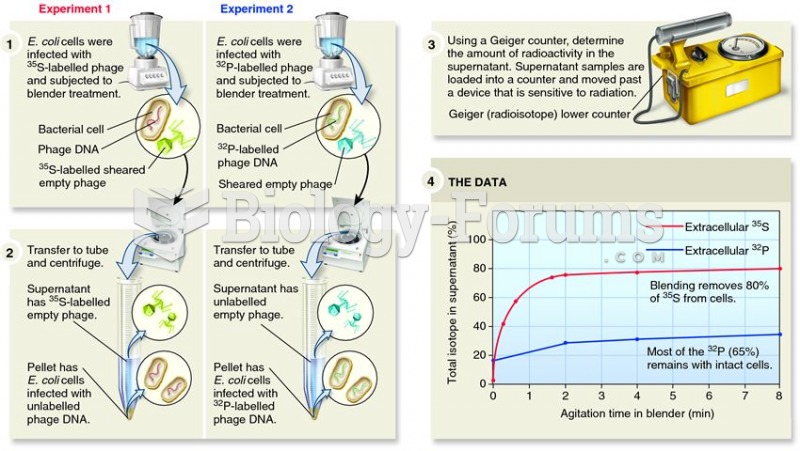
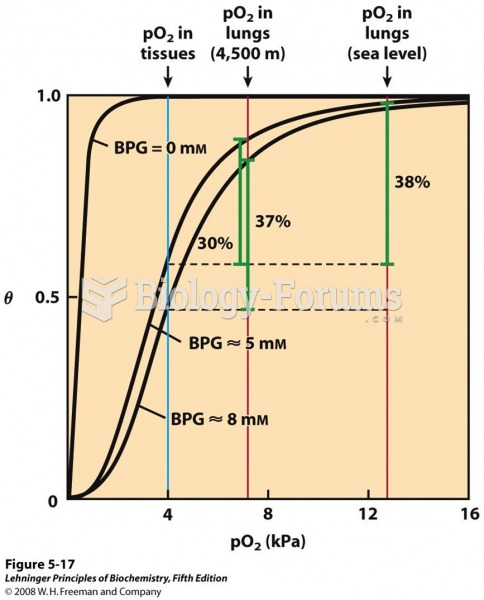
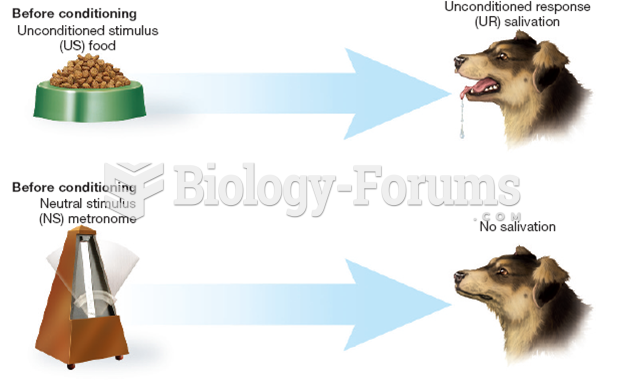
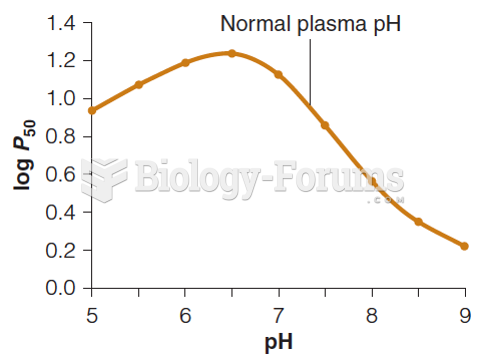
|
|
|
Excessive alcohol use costs the country approximately $235 billion every year.
All patients with hyperparathyroidism will develop osteoporosis. The parathyroid glands maintain blood calcium within the normal range. All patients with this disease will continue to lose calcium from their bones every day, and there is no way to prevent the development of osteoporosis as a result.
Persons who overdose with cardiac glycosides have a better chance of overall survival if they can survive the first 24 hours after the overdose.
The strongest synthetic topical retinoid drug available, tazarotene, is used to treat sun-damaged skin, acne, and psoriasis.
Russia has the highest death rate from cardiovascular disease followed by the Ukraine, Romania, Hungary, and Poland.