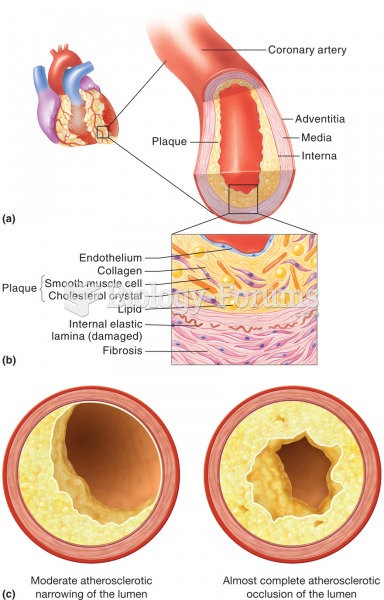
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Asthma cases in Americans are about 75% higher today than they were in 1980.
Did you know?
Aspirin is the most widely used drug in the world. It has even been recognized as such by the Guinness Book of World Records.
Did you know?
A strange skin disease referred to as Morgellons has occurred in the southern United States and in California. Symptoms include slowly healing sores, joint pain, persistent fatigue, and a sensation of things crawling through the skin. Another symptom is strange-looking, threadlike extrusions coming out of the skin.
Did you know?
Multiple experimental evidences have confirmed that at the molecular level, cancer is caused by lesions in cellular DNA.
Did you know?
Green tea is able to stop the scent of garlic or onion from causing bad breath.