|
|
|
Did you know?
Studies show that systolic blood pressure can be significantly lowered by taking statins. In fact, the higher the patient's baseline blood pressure, the greater the effect of statins on his or her blood pressure.
Did you know?
Women are 50% to 75% more likely than men to experience an adverse drug reaction.
Did you know?
The first oral chemotherapy drug for colon cancer was approved by FDA in 2001.
Did you know?
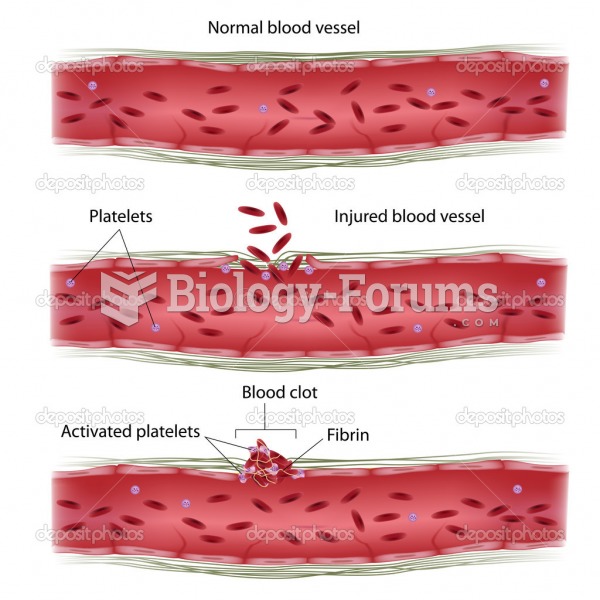
Blood is approximately twice as thick as water because of the cells and other components found in it.
Did you know?
Thyroid conditions may make getting pregnant impossible.