This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
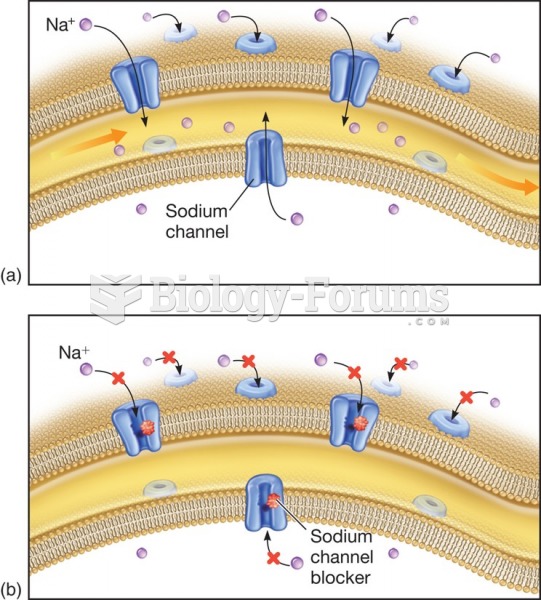
Many of the drugs used by neuroscientists are derived from toxic plants and venomous animals (such as snakes, spiders, snails, and puffer fish).
Did you know?
The most common childhood diseases include croup, chickenpox, ear infections, flu, pneumonia, ringworm, respiratory syncytial virus, scabies, head lice, and asthma.
Did you know?
There are more sensory neurons in the tongue than in any other part of the body.
Did you know?
The newest statin drug, rosuvastatin, has been called a superstatin because it appears to reduce LDL cholesterol to a greater degree than the other approved statin drugs.
Did you know?
Urine turns bright yellow if larger than normal amounts of certain substances are consumed; one of these substances is asparagus.