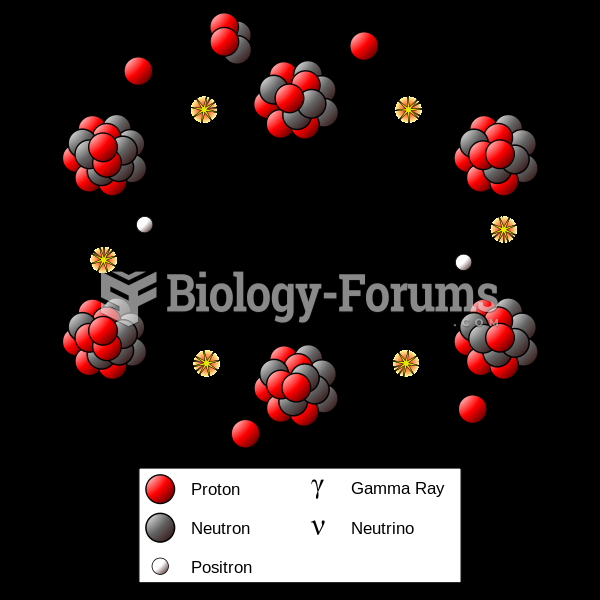
|
|
|
All adults should have their cholesterol levels checked once every 5 years. During 2009–2010, 69.4% of Americans age 20 and older reported having their cholesterol checked within the last five years.
People about to have surgery must tell their health care providers about all supplements they take.
Bisphosphonates were first developed in the nineteenth century. They were first investigated for use in disorders of bone metabolism in the 1960s. They are now used clinically for the treatment of osteoporosis, Paget's disease, bone metastasis, multiple myeloma, and other conditions that feature bone fragility.
Green tea is able to stop the scent of garlic or onion from causing bad breath.
It is difficult to obtain enough calcium without consuming milk or other dairy foods.