This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
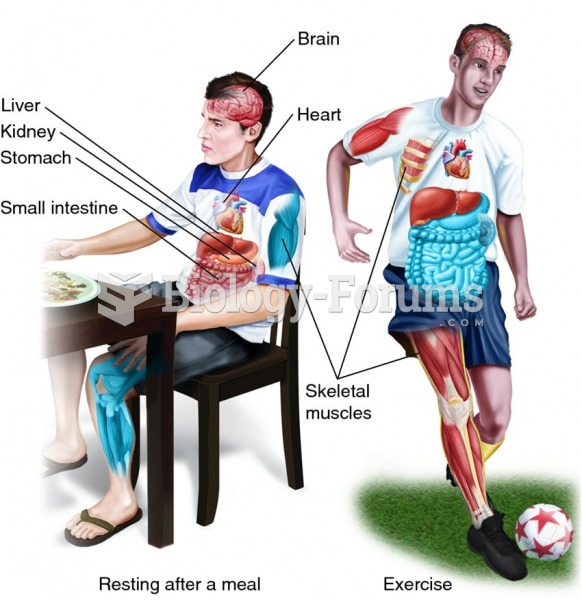
Drug-induced pharmacodynamic effects manifested in older adults include drug-induced renal toxicity, which can be a major factor when these adults are experiencing other kidney problems.
Did you know?
Approximately 500,000 babies are born each year in the United States to teenage mothers.
Did you know?
Atropine was named after the Greek goddess Atropos, the oldest and ugliest of the three sisters known as the Fates, who controlled the destiny of men.
Did you know?
Fewer than 10% of babies are born on their exact due dates, 50% are born within 1 week of the due date, and 90% are born within 2 weeks of the date.
Did you know?
About 100 new prescription or over-the-counter drugs come into the U.S. market every year.