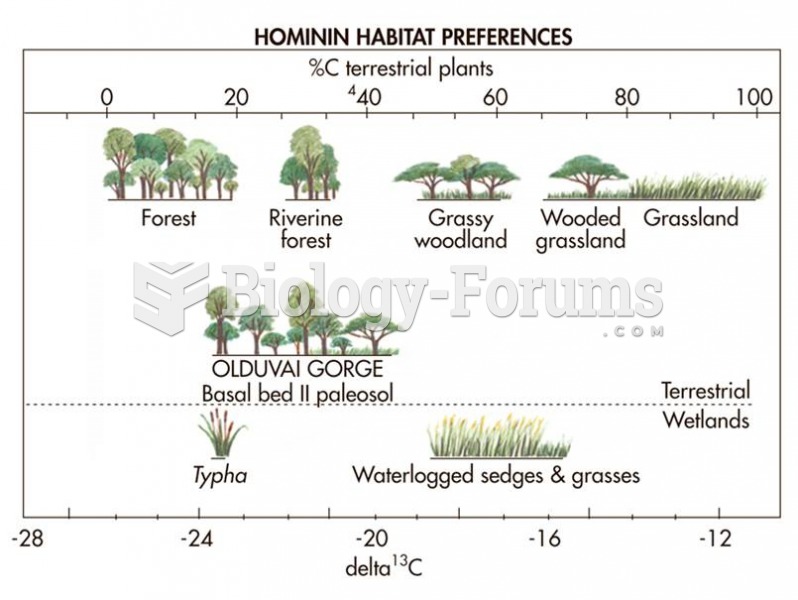
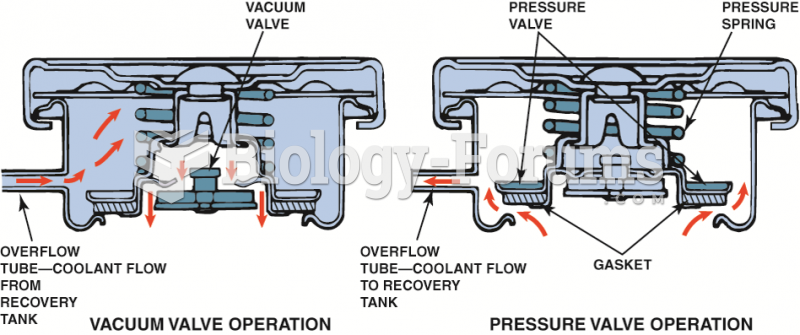
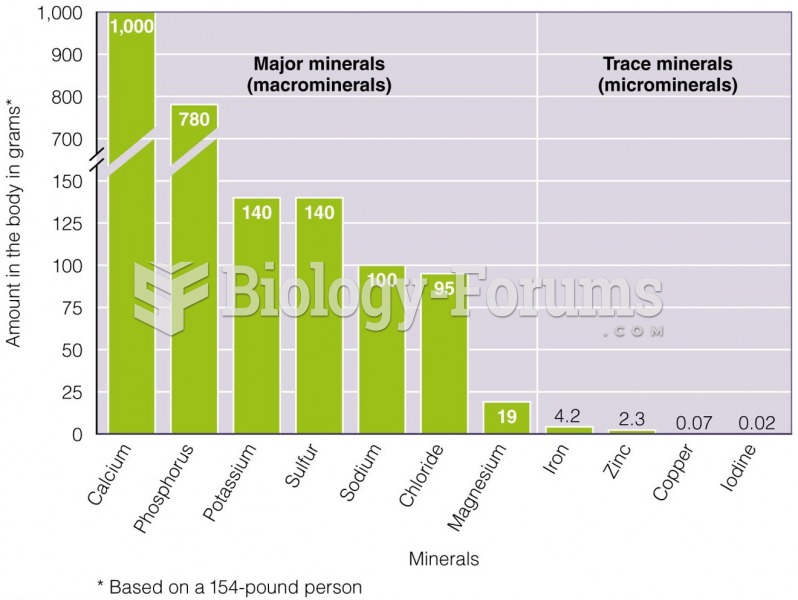
|
|
|
Your heart beats over 36 million times a year.
The FDA recognizes 118 routes of administration.
Cocaine was isolated in 1860 and first used as a local anesthetic in 1884. Its first clinical use was by Sigmund Freud to wean a patient from morphine addiction. The fictional character Sherlock Holmes was supposed to be addicted to cocaine by injection.
A strange skin disease referred to as Morgellons has occurred in the southern United States and in California. Symptoms include slowly healing sores, joint pain, persistent fatigue, and a sensation of things crawling through the skin. Another symptom is strange-looking, threadlike extrusions coming out of the skin.
Though “Krazy Glue” or “Super Glue” has the ability to seal small wounds, it is not recommended for this purpose since it contains many substances that should not enter the body through the skin, and may be harmful.