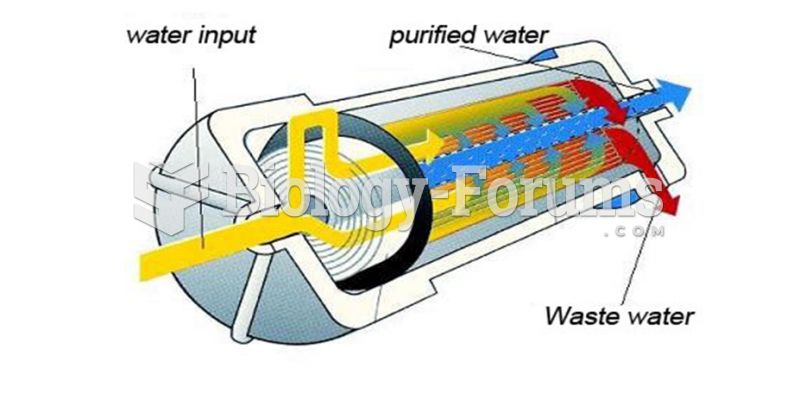
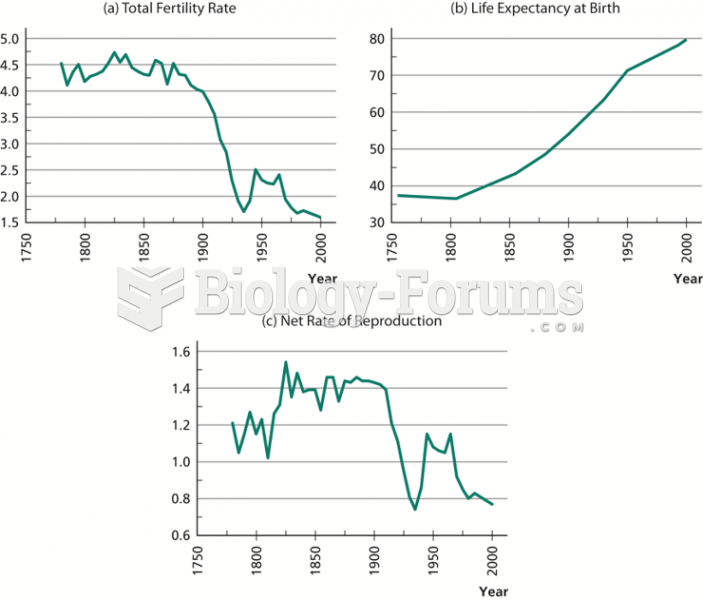
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Amphetamine poisoning can cause intravascular coagulation, circulatory collapse, rhabdomyolysis, ischemic colitis, acute psychosis, hyperthermia, respiratory distress syndrome, and pericarditis.
Did you know?
The ratio of hydrogen atoms to oxygen in water (H2O) is 2:1.
Did you know?
More than 34,000 trademarked medication names and more than 10,000 generic medication names are in use in the United States.
Did you know?
The liver is the only organ that has the ability to regenerate itself after certain types of damage. As much as 25% of the liver can be removed, and it will still regenerate back to its original shape and size. However, the liver cannot regenerate after severe damage caused by alcohol.
Did you know?
Green tea is able to stop the scent of garlic or onion from causing bad breath.