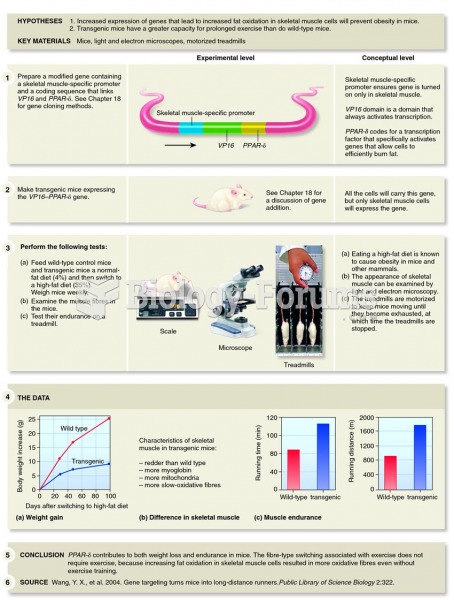
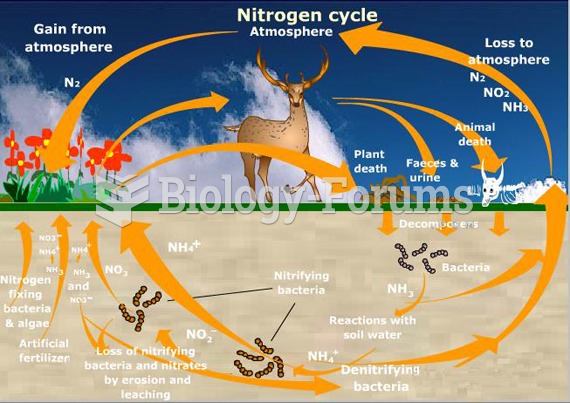
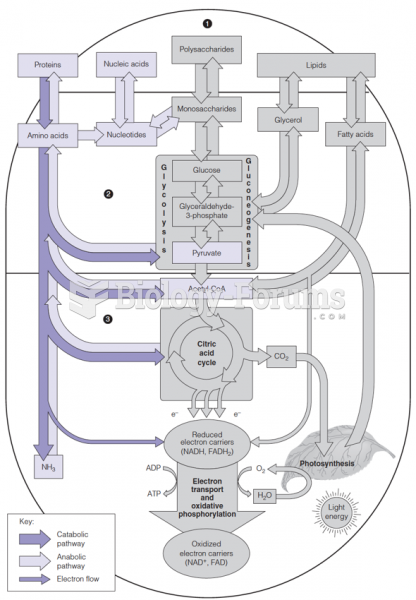
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Carbamazepine can interfere with the results of home pregnancy tests. If you are taking carbamazepine, do not try to test for pregnancy at home.
Did you know?
The calories found in one piece of cherry cheesecake could light a 60-watt light bulb for 1.5 hours.
Did you know?
Approximately 15–25% of recognized pregnancies end in miscarriage. However, many miscarriages often occur before a woman even knows she is pregnant.
Did you know?
The strongest synthetic topical retinoid drug available, tazarotene, is used to treat sun-damaged skin, acne, and psoriasis.
Did you know?
In inpatient settings, adverse drug events account for an estimated one in three of all hospital adverse events. They affect approximately 2 million hospital stays every year, and prolong hospital stays by between one and five days.