|
|
|
Bacteria have been found alive in a lake buried one half mile under ice in Antarctica.
Of the estimated 2 million heroin users in the United States, 600,000–800,000 are considered hardcore addicts. Heroin addiction is considered to be one of the hardest addictions to recover from.
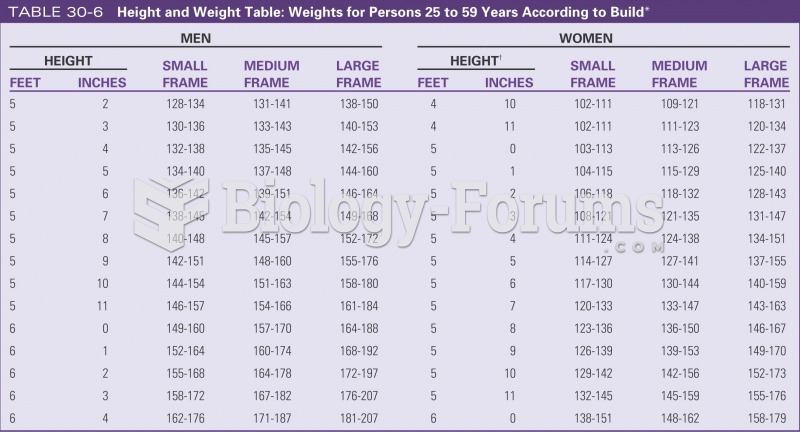
An identified risk factor for osteoporosis is the intake of excessive amounts of vitamin A. Dietary intake of approximately double the recommended daily amount of vitamin A, by women, has been shown to reduce bone mineral density and increase the chances for hip fractures compared with women who consumed the recommended daily amount (or less) of vitamin A.
The longest a person has survived after a heart transplant is 24 years.
Blood is approximately twice as thick as water because of the cells and other components found in it.