This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Pregnant women usually experience a heightened sense of smell beginning late in the first trimester. Some experts call this the body's way of protecting a pregnant woman from foods that are unsafe for the fetus.
Did you know?
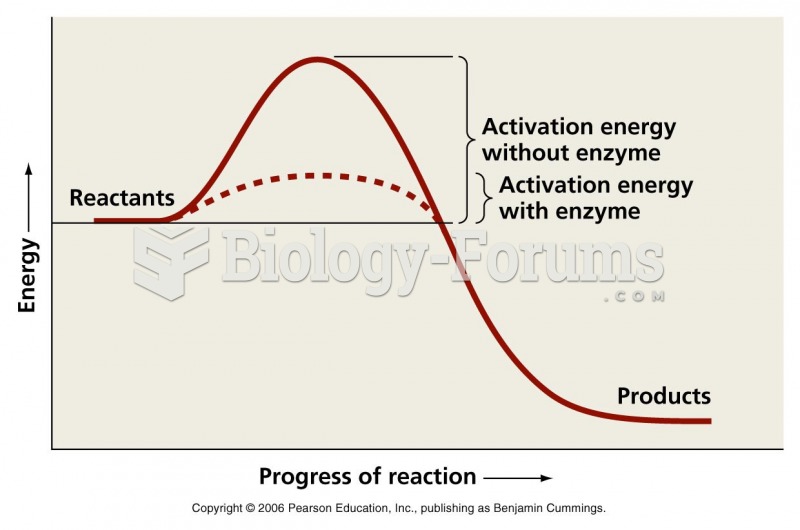
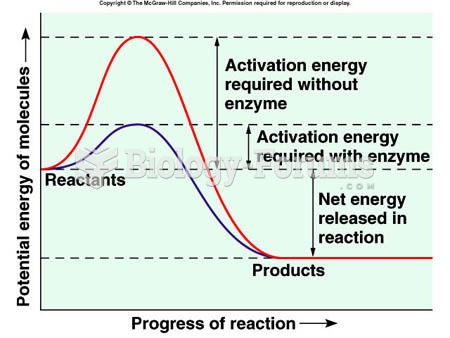
The term pharmacology is derived from the Greek words pharmakon("claim, medicine, poison, or remedy") and logos ("study").
Did you know?
There are 60,000 miles of blood vessels in every adult human.
Did you know?
There are more sensory neurons in the tongue than in any other part of the body.
Did you know?
Malaria was not eliminated in the United States until 1951. The term eliminated means that no new cases arise in a country for 3 years.