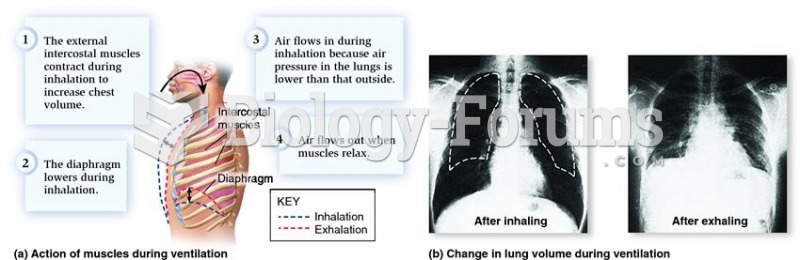
|
|
|
A headache when you wake up in the morning is indicative of sinusitis. Other symptoms of sinusitis can include fever, weakness, tiredness, a cough that may be more severe at night, and a runny nose or nasal congestion.
There used to be a metric calendar, as well as metric clocks. The metric calendar, or "French Republican Calendar" divided the year into 12 months, but each month was divided into three 10-day weeks. Each day had 10 decimal hours. Each hour had 100 decimal minutes. Due to lack of popularity, the metric clocks and calendars were ended in 1795, three years after they had been first marketed.
The human body's pharmacokinetics are quite varied. Our hair holds onto drugs longer than our urine, blood, or saliva. For example, alcohol can be detected in the hair for up to 90 days after it was consumed. The same is true for marijuana, cocaine, ecstasy, heroin, methamphetamine, and nicotine.
The first monoclonal antibodies were made exclusively from mouse cells. Some are now fully human, which means they are likely to be safer and may be more effective than older monoclonal antibodies.
Vaccines prevent between 2.5 and 4 million deaths every year.
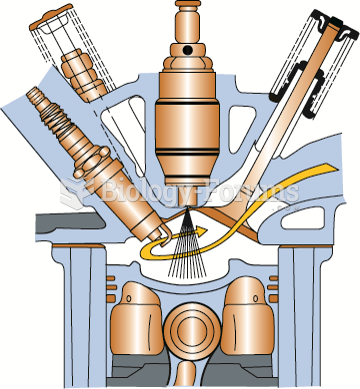 A gasoline direct-injection system injects fuel under high pressure directly into the combustion ...
A gasoline direct-injection system injects fuel under high pressure directly into the combustion ...
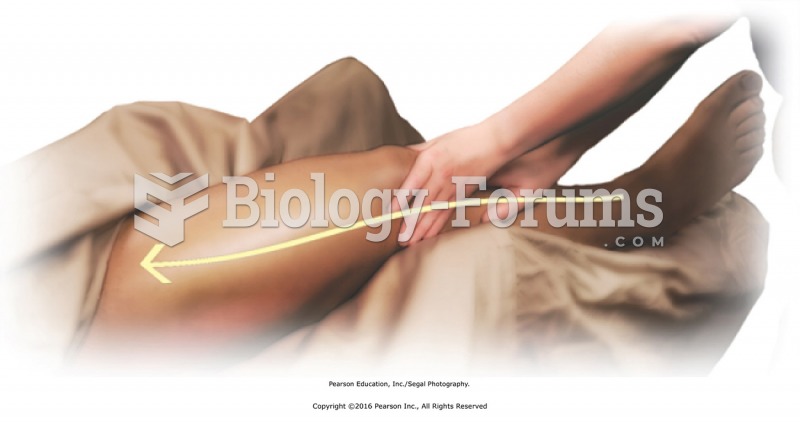 Apply lubricant to the entire right limb using basic sliding effleurage. Apply moderate pressure ...
Apply lubricant to the entire right limb using basic sliding effleurage. Apply moderate pressure ...