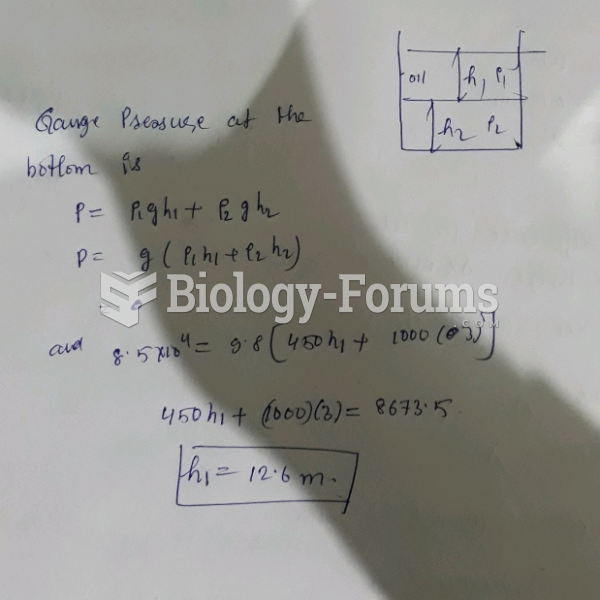
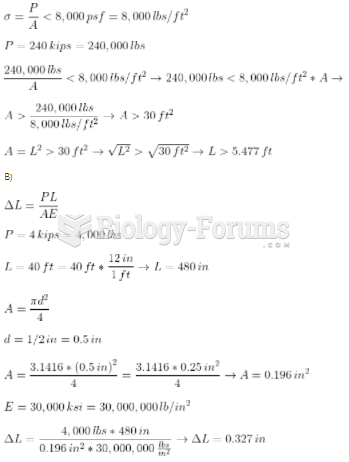
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
When blood is exposed to air, it clots. Heparin allows the blood to come in direct contact with air without clotting.
Did you know?
More than one-third of adult Americans are obese. Diseases that kill the largest number of people annually, such as heart disease, cancer, diabetes, stroke, and hypertension, can be attributed to diet.
Did you know?
There are 60,000 miles of blood vessels in every adult human.
Did you know?
Cyanide works by making the human body unable to use oxygen.
Did you know?
The newest statin drug, rosuvastatin, has been called a superstatin because it appears to reduce LDL cholesterol to a greater degree than the other approved statin drugs.