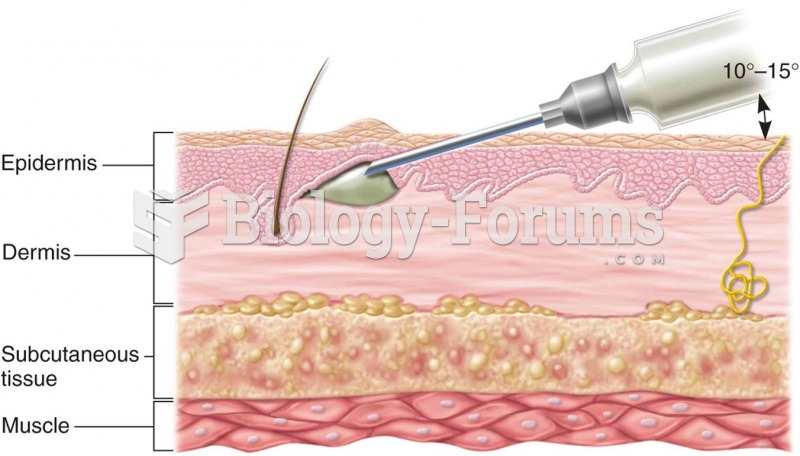
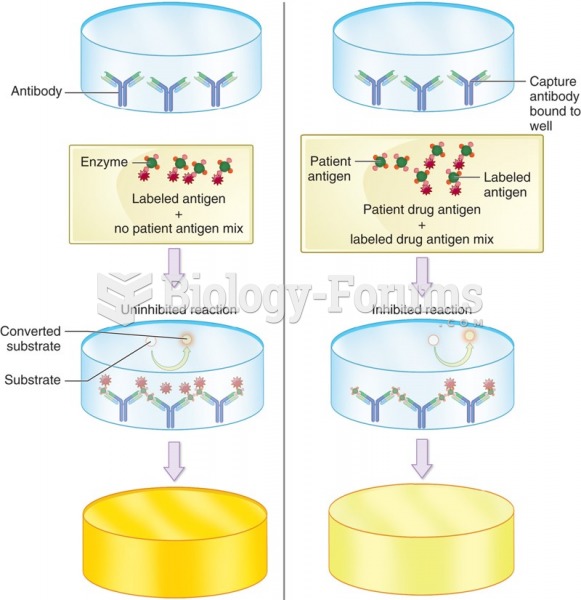
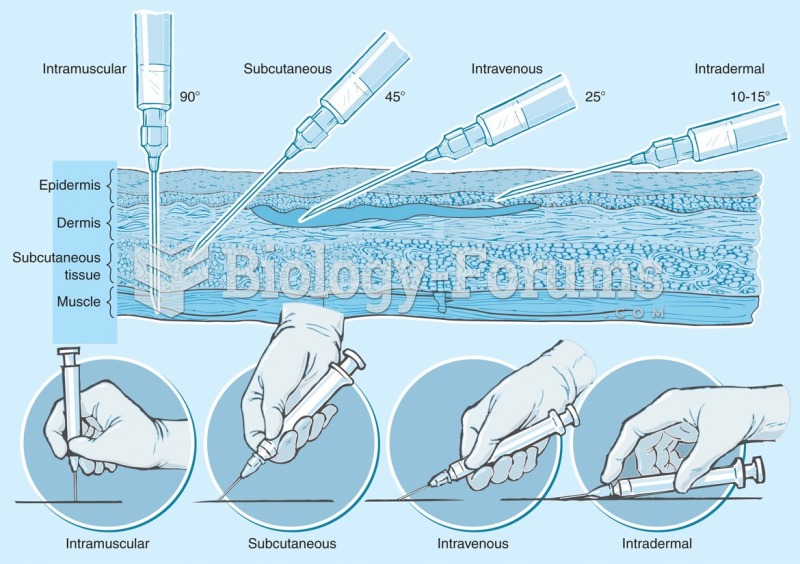
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
There are 60,000 miles of blood vessels in every adult human.
Did you know?
People with high total cholesterol have about two times the risk for heart disease as people with ideal levels.
Did you know?
Drug-induced pharmacodynamic effects manifested in older adults include drug-induced renal toxicity, which can be a major factor when these adults are experiencing other kidney problems.
Did you know?
The longest a person has survived after a heart transplant is 24 years.
Did you know?
Approximately one in four people diagnosed with diabetes will develop foot problems. Of these, about one-third will require lower extremity amputation.