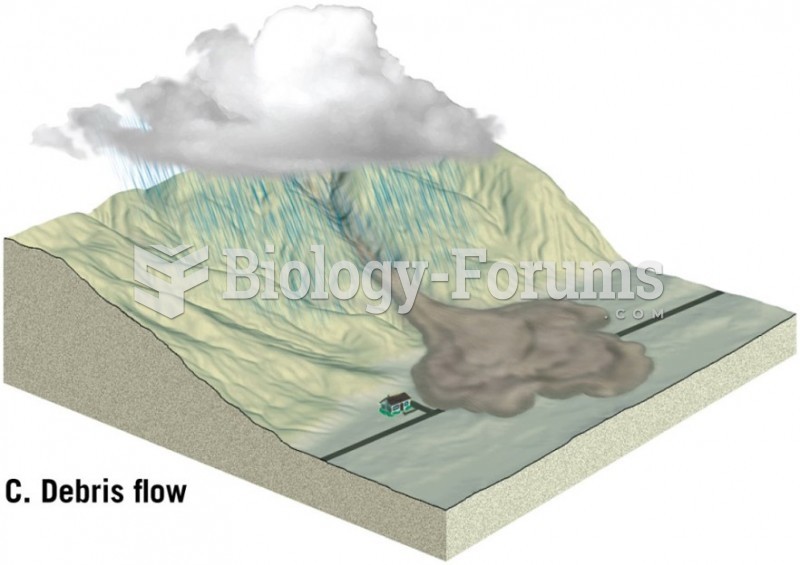
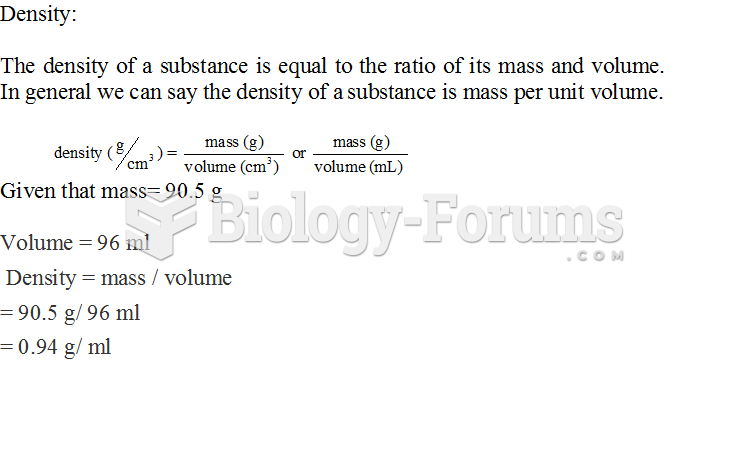
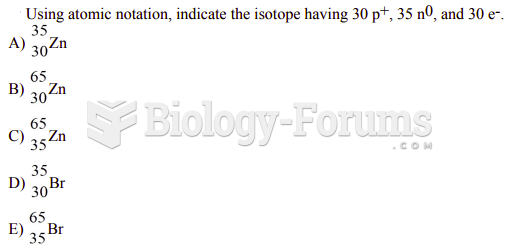
|
|
|
Cutaneous mucormycosis is a rare fungal infection that has been fatal in at least 29% of cases, and in as many as 83% of cases, depending on the patient's health prior to infection. It has occurred often after natural disasters such as tornados, and early treatment is essential.
Although not all of the following muscle groups are commonly used, intramuscular injections may be given into the abdominals, biceps, calves, deltoids, gluteals, laterals, pectorals, quadriceps, trapezoids, and triceps.
It is believed that the Incas used anesthesia. Evidence supports the theory that shamans chewed cocoa leaves and drilled holes into the heads of patients (letting evil spirits escape), spitting into the wounds they made. The mixture of cocaine, saliva, and resin numbed the site enough to allow hours of drilling.
Blood in the urine can be a sign of a kidney stone, glomerulonephritis, or other kidney problems.
Recent studies have shown that the number of medication errors increases in relation to the number of orders that are verified per pharmacist, per work shift.
 Prostate cancer. In this example, a large mass has grown into the urinary bladder. Prostate cancer i
Prostate cancer. In this example, a large mass has grown into the urinary bladder. Prostate cancer i
 Internal structures of main sequence stars, convection zones with arrowed cycles and radiative zones
Internal structures of main sequence stars, convection zones with arrowed cycles and radiative zones