|
|
|
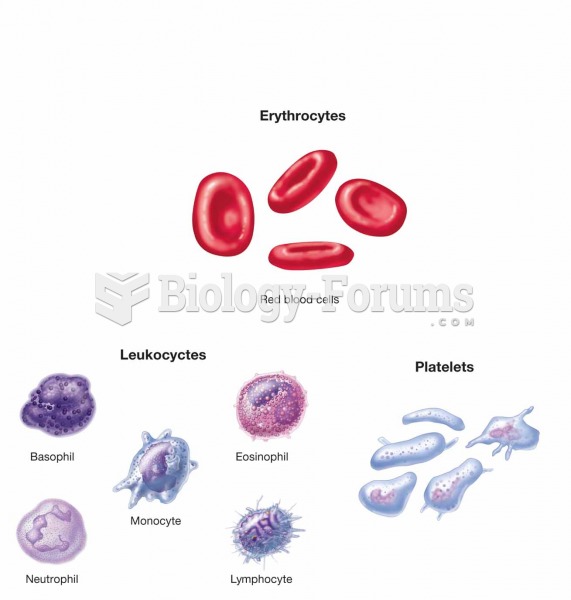
There are over 65,000 known species of protozoa. About 10,000 species are parasitic.
Always store hazardous household chemicals in their original containers out of reach of children. These include bleach, paint, strippers and products containing turpentine, garden chemicals, oven cleaners, fondue fuels, nail polish, and nail polish remover.
By definition, when a medication is administered intravenously, its bioavailability is 100%.
Oliver Wendell Holmes is credited with introducing the words "anesthesia" and "anesthetic" into the English language in 1846.
Chronic necrotizing aspergillosis has a slowly progressive process that, unlike invasive aspergillosis, does not spread to other organ systems or the blood vessels. It most often affects middle-aged and elderly individuals, spreading to surrounding tissue in the lungs. The disease often does not respond to conventionally successful treatments, and requires individualized therapies in order to keep it from becoming life-threatening.
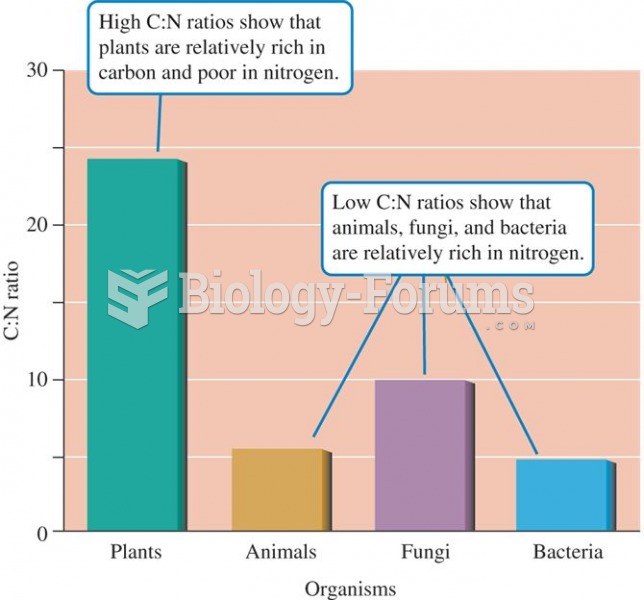 On average, the ratio of carbon to nitrogen is much higher in terrestrial plants than in other major
On average, the ratio of carbon to nitrogen is much higher in terrestrial plants than in other major
 Bow shock formed by the magnetosphere of LL Orionis (center) as it collides with the Orion Nebula fl
Bow shock formed by the magnetosphere of LL Orionis (center) as it collides with the Orion Nebula fl