This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Everyone has one nostril that is larger than the other.
Did you know?
Coca-Cola originally used coca leaves and caffeine from the African kola nut. It was advertised as a therapeutic agent and "pickerupper." Eventually, its formulation was changed, and the coca leaves were removed because of the effects of regulation on cocaine-related products.
Did you know?
Your heart beats over 36 million times a year.
Did you know?
Famous people who died from poisoning or drug overdose include, Adolf Hitler, Socrates, Juan Ponce de Leon, Marilyn Monroe, Judy Garland, and John Belushi.
Did you know?
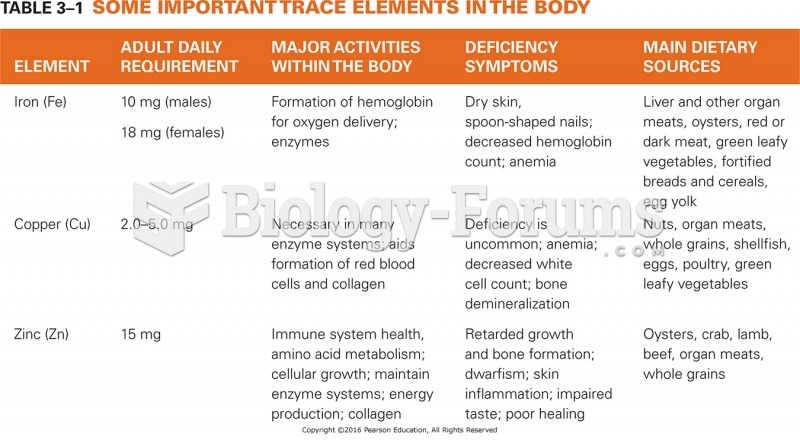
The B-complex vitamins and vitamin C are not stored in the body and must be replaced each day.