|
|
|
Cocaine was isolated in 1860 and first used as a local anesthetic in 1884. Its first clinical use was by Sigmund Freud to wean a patient from morphine addiction. The fictional character Sherlock Holmes was supposed to be addicted to cocaine by injection.
There are major differences in the metabolism of morphine and the illegal drug heroin. Morphine mostly produces its CNS effects through m-receptors, and at k- and d-receptors. Heroin has a slight affinity for opiate receptors. Most of its actions are due to metabolism to active metabolites (6-acetylmorphine, morphine, and morphine-6-glucuronide).
In ancient Rome, many of the richer people in the population had lead-induced gout. The reason for this is unclear. Lead poisoning has also been linked to madness.
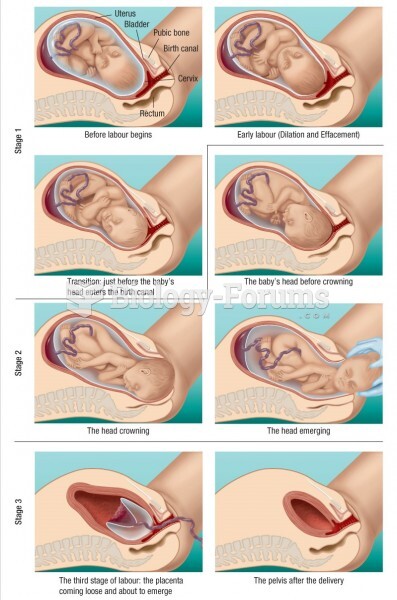
Approximately 500,000 babies are born each year in the United States to teenage mothers.
Lower drug doses for elderly patients should be used first, with titrations of the dose as tolerated to prevent unwanted drug-related pharmacodynamic effects.
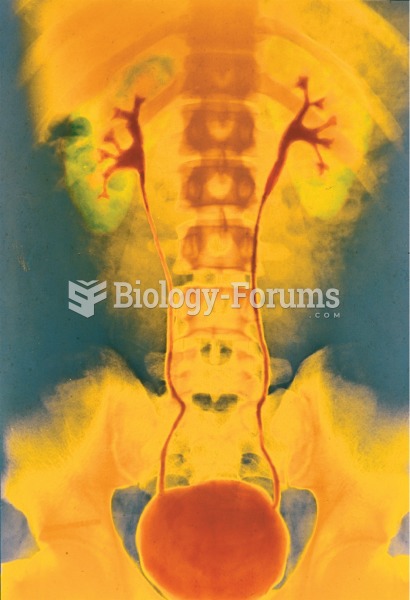 Retrograde pyelogram. A contrast medium is injected into the ureter using a cystoscope, and the X-ra
Retrograde pyelogram. A contrast medium is injected into the ureter using a cystoscope, and the X-ra
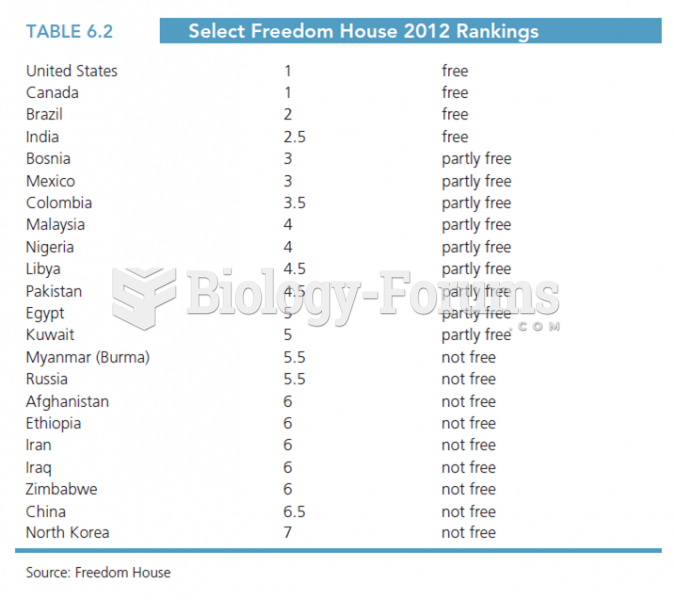 Freedom House is a nonprofit institution that uses several different factors to rank countries based
Freedom House is a nonprofit institution that uses several different factors to rank countries based