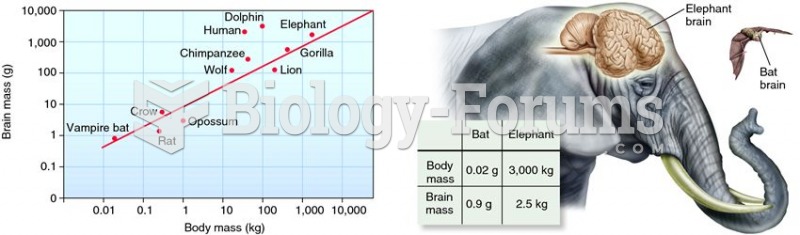
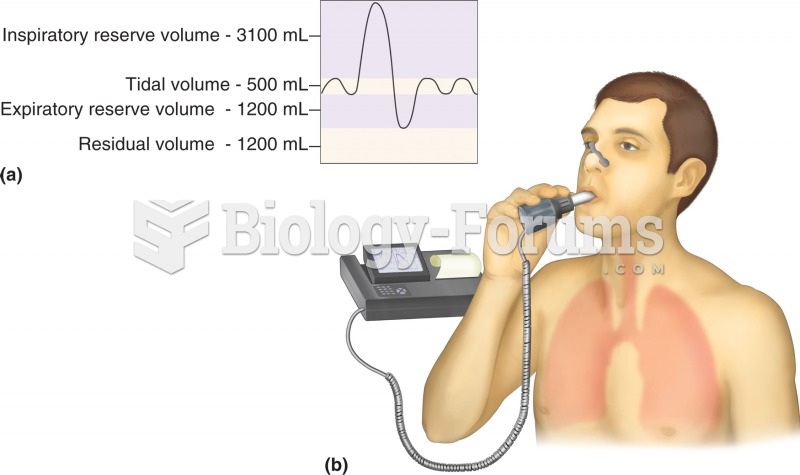
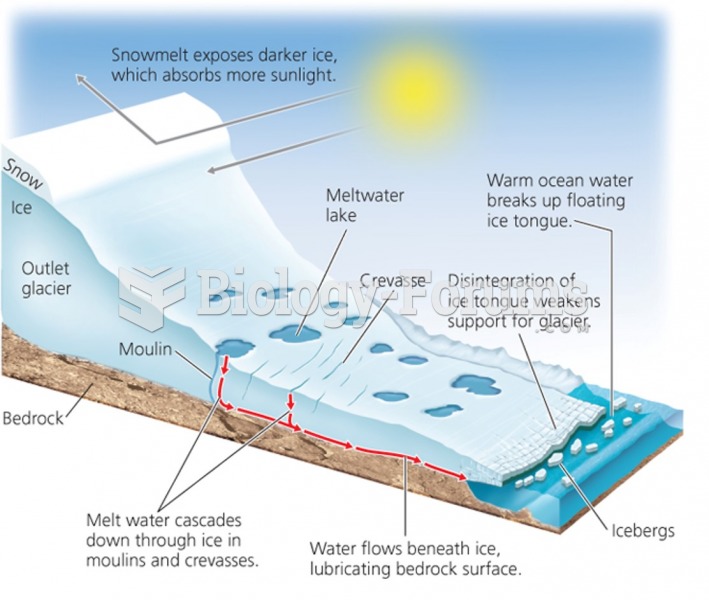
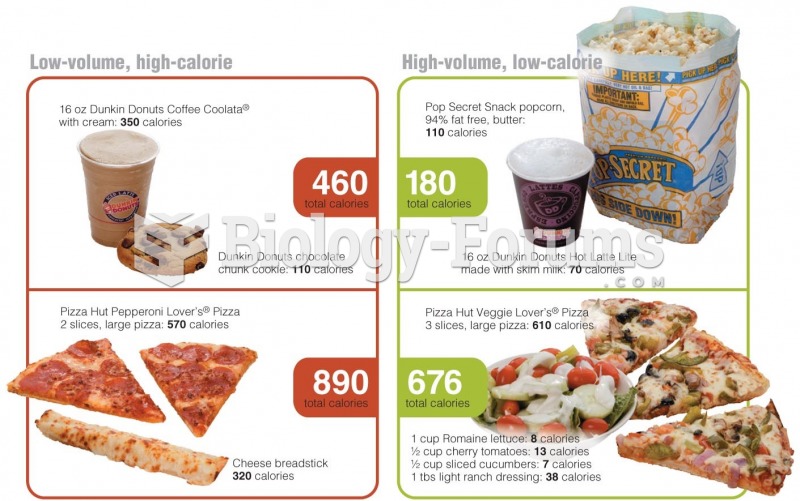
|
|
|
Certain chemicals, after ingestion, can be converted by the body into cyanide. Most of these chemicals have been removed from the market, but some old nail polish remover, solvents, and plastics manufacturing solutions can contain these substances.
Pubic lice (crabs) are usually spread through sexual contact. You cannot catch them by using a public toilet.
In 1885, the Lloyd Manufacturing Company of Albany, New York, promoted and sold "Cocaine Toothache Drops" at 15 cents per bottle! In 1914, the Harrison Narcotic Act brought the sale and distribution of this drug under federal control.
The first documented use of surgical anesthesia in the United States was in Connecticut in 1844.
The familiar sounds of your heart are made by the heart's valves as they open and close.