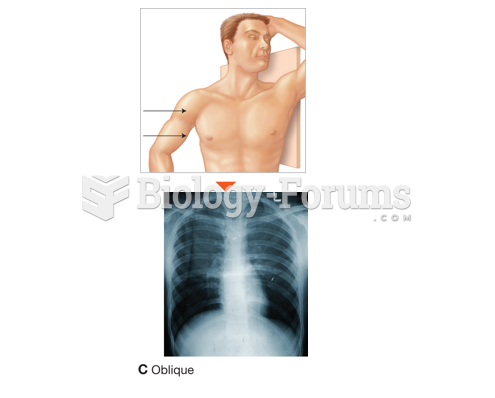
|
|
|
Patients should never assume they are being given the appropriate drugs. They should make sure they know which drugs are being prescribed, and always double-check that the drugs received match the prescription.
Approximately 500,000 babies are born each year in the United States to teenage mothers.
Colchicine is a highly poisonous alkaloid originally extracted from a type of saffron plant that is used mainly to treat gout.
Coca-Cola originally used coca leaves and caffeine from the African kola nut. It was advertised as a therapeutic agent and "pickerupper." Eventually, its formulation was changed, and the coca leaves were removed because of the effects of regulation on cocaine-related products.
Hypertension is a silent killer because it is deadly and has no significant early symptoms. The danger from hypertension is the extra load on the heart, which can lead to hypertensive heart disease and kidney damage. This occurs without any major symptoms until the high blood pressure becomes extreme. Regular blood pressure checks are an important method of catching hypertension before it can kill you.
 Dred Scott and his wife and children are featured on the cover of Frank Leslie’s Illustrated Newspap
Dred Scott and his wife and children are featured on the cover of Frank Leslie’s Illustrated Newspap
 Durkheim believed that modern societies produce feelings of isolation, much of which come from the ...
Durkheim believed that modern societies produce feelings of isolation, much of which come from the ...