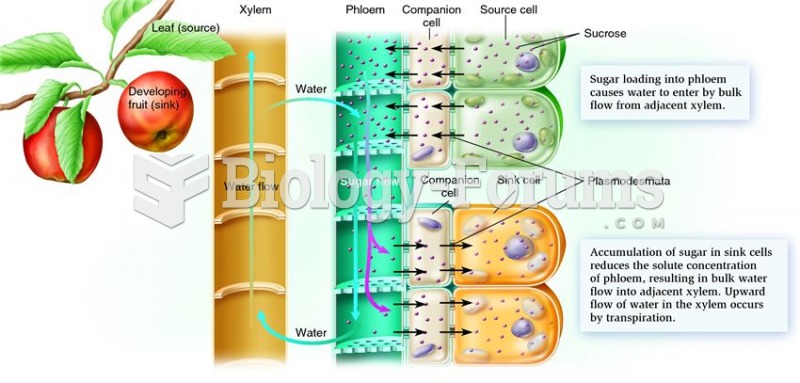
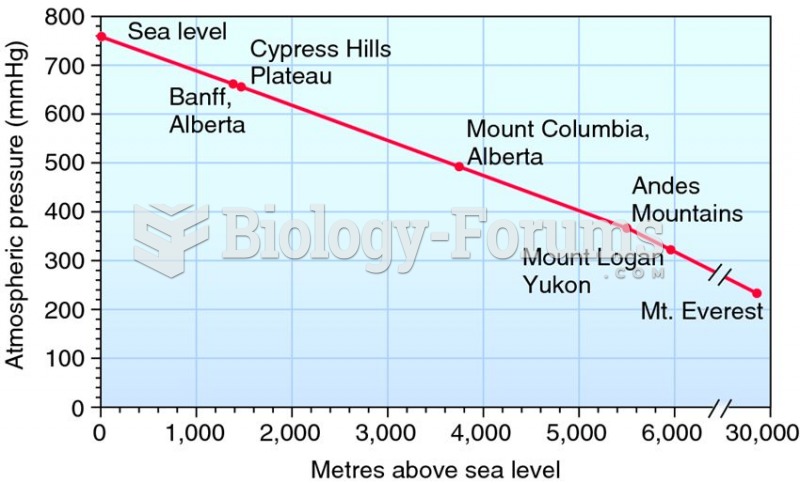
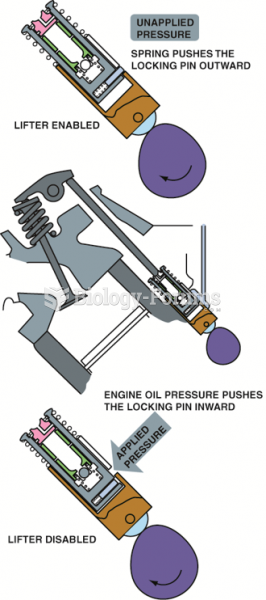
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
The U.S. Pharmacopeia Medication Errors Reporting Program states that approximately 50% of all medication errors involve insulin.
Did you know?
Women are 50% to 75% more likely than men to experience an adverse drug reaction.
Did you know?
More than nineteen million Americans carry the factor V gene that causes blood clots, pulmonary embolism, and heart disease.
Did you know?
If you could remove all of your skin, it would weigh up to 5 pounds.
Did you know?
Patients who have undergone chemotherapy for the treatment of cancer often complain of a lack of mental focus; memory loss; and a general diminution in abilities such as multitasking, attention span, and general mental agility.