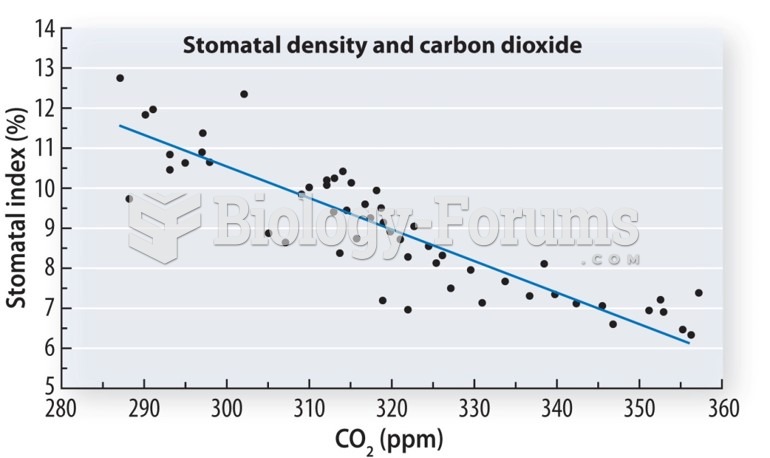
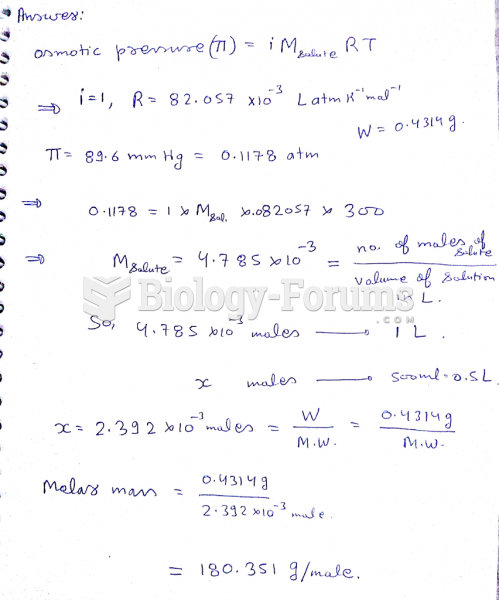
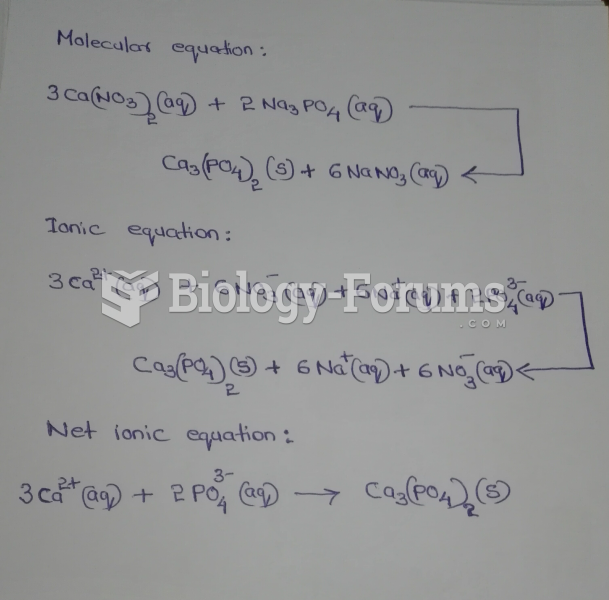
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Long-term mental and physical effects from substance abuse include: paranoia, psychosis, immune deficiencies, and organ damage.
Did you know?
Green tea is able to stop the scent of garlic or onion from causing bad breath.
Did you know?
Thyroid conditions may make getting pregnant impossible.
Did you know?
Colchicine is a highly poisonous alkaloid originally extracted from a type of saffron plant that is used mainly to treat gout.
Did you know?
Excessive alcohol use costs the country approximately $235 billion every year.