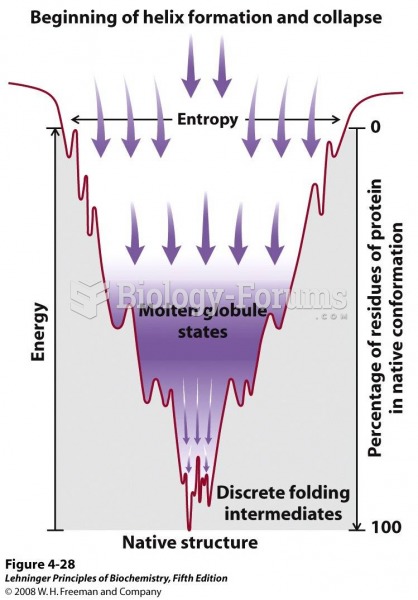
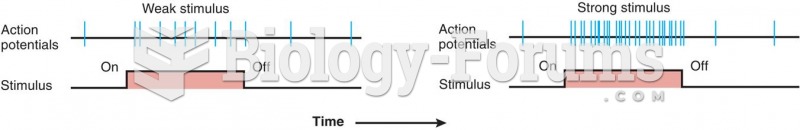
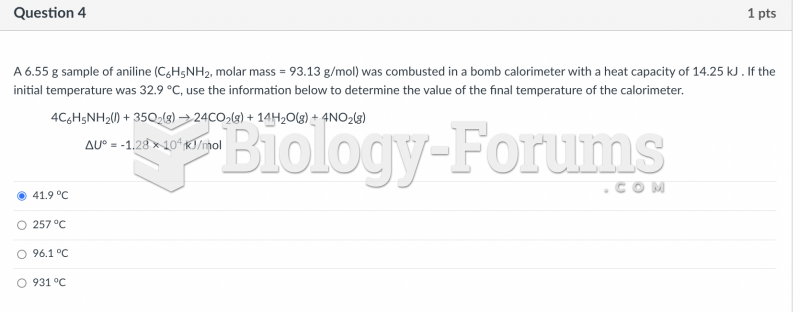
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
The longest a person has survived after a heart transplant is 24 years.
Did you know?
The Romans did not use numerals to indicate fractions but instead used words to indicate parts of a whole.
Did you know?
The lipid bilayer is made of phospholipids. They are arranged in a double layer because one of their ends is attracted to water while the other is repelled by water.
Did you know?
No drugs are available to relieve parathyroid disease. Parathyroid disease is caused by a parathyroid tumor, and it needs to be removed by surgery.
Did you know?
The FDA recognizes 118 routes of administration.