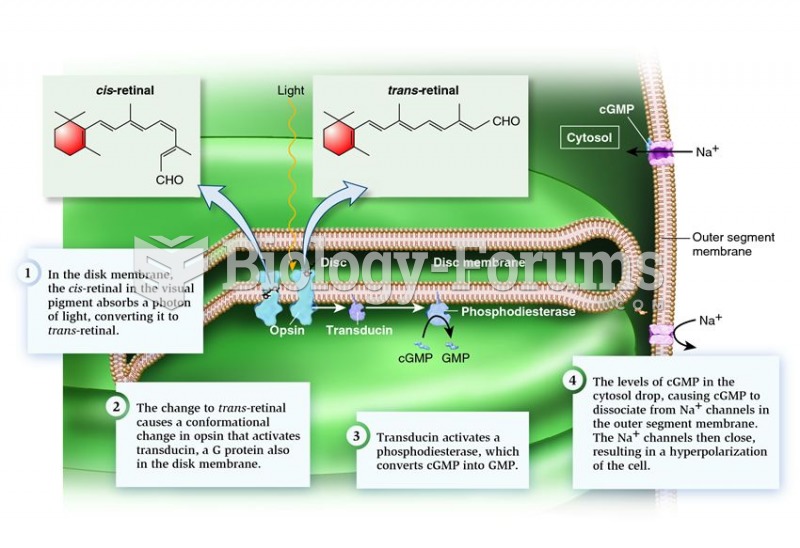
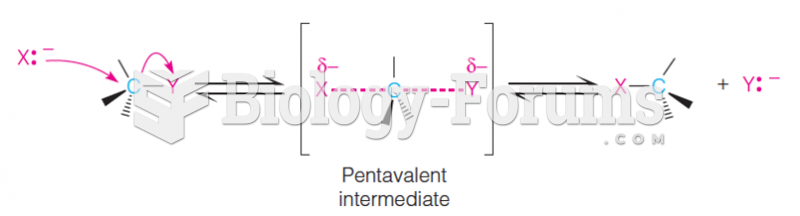
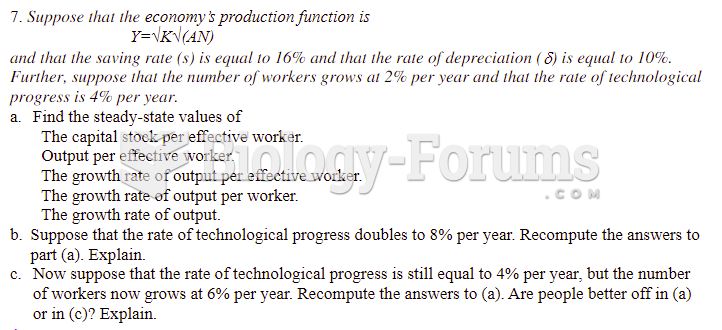
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
There are 60,000 miles of blood vessels in every adult human.
Did you know?
Acute bronchitis is an inflammation of the breathing tubes (bronchi), which causes increased mucus production and other changes. It is usually caused by bacteria or viruses, can be serious in people who have pulmonary or cardiac diseases, and can lead to pneumonia.
Did you know?
Serum cholesterol testing in adults is recommended every 1 to 5 years. People with diabetes and a family history of high cholesterol should be tested even more frequently.
Did you know?
Cytomegalovirus affects nearly the same amount of newborns every year as Down syndrome.
Did you know?
Multiple sclerosis is a condition wherein the body's nervous system is weakened by an autoimmune reaction that attacks the myelin sheaths of neurons.