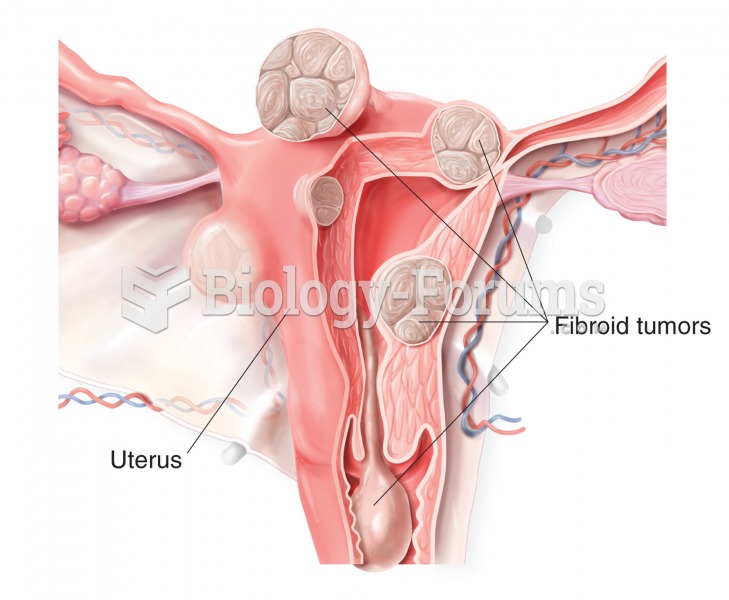
|
|
|
This year, an estimated 1.4 million Americans will have a new or recurrent heart attack.
Astigmatism is the most common vision problem. It may accompany nearsightedness or farsightedness. It is usually caused by an irregularly shaped cornea, but sometimes it is the result of an irregularly shaped lens. Either type can be corrected by eyeglasses, contact lenses, or refractive surgery.
The average office desk has 400 times more bacteria on it than a toilet.
Throughout history, plants containing cardiac steroids have been used as heart drugs and as poisons (e.g., in arrows used in combat), emetics, and diuretics.
The liver is the only organ that has the ability to regenerate itself after certain types of damage. As much as 25% of the liver can be removed, and it will still regenerate back to its original shape and size. However, the liver cannot regenerate after severe damage caused by alcohol.
 Seasonal changes in biomass and growth form of benthic algae in the Eel River, California: (a) in ea
Seasonal changes in biomass and growth form of benthic algae in the Eel River, California: (a) in ea
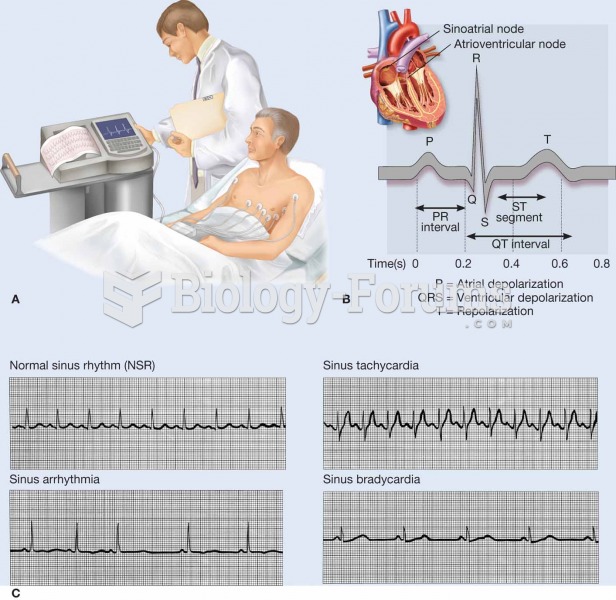 An electrocardiogram (ECG, EKG) is a commonly used procedure in which the electrical events associat
An electrocardiogram (ECG, EKG) is a commonly used procedure in which the electrical events associat