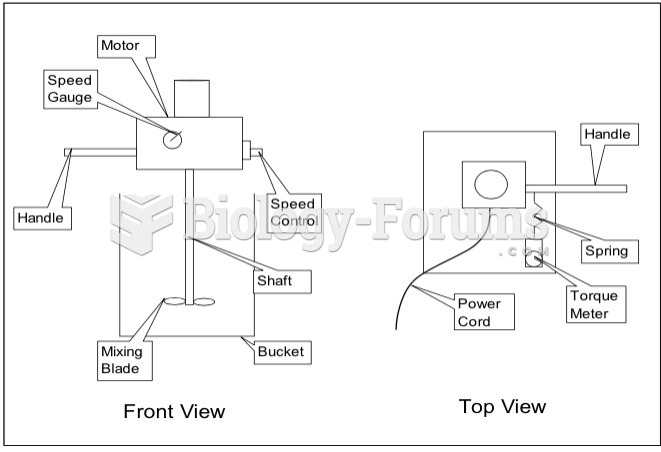
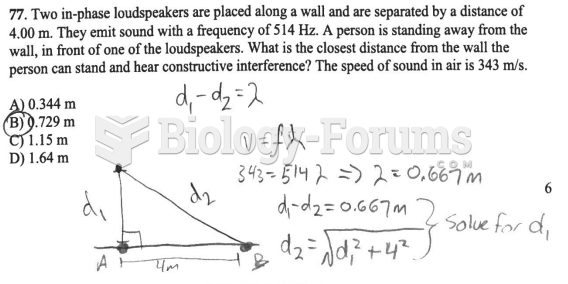
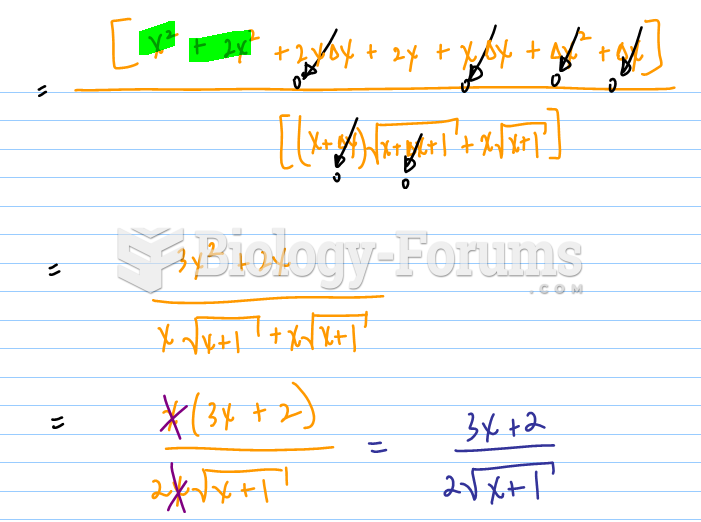
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
In 1844, Charles Goodyear obtained the first patent for a rubber condom.
Did you know?
Critical care patients are twice as likely to receive the wrong medication. Of these errors, 20% are life-threatening, and 42% require additional life-sustaining treatments.
Did you know?
Vaccines prevent between 2.5 and 4 million deaths every year.
Did you know?
The shortest mature adult human of whom there is independent evidence was Gul Mohammed in India. In 1990, he was measured in New Delhi and stood 22.5 inches tall.
Did you know?
Lower drug doses for elderly patients should be used first, with titrations of the dose as tolerated to prevent unwanted drug-related pharmacodynamic effects.