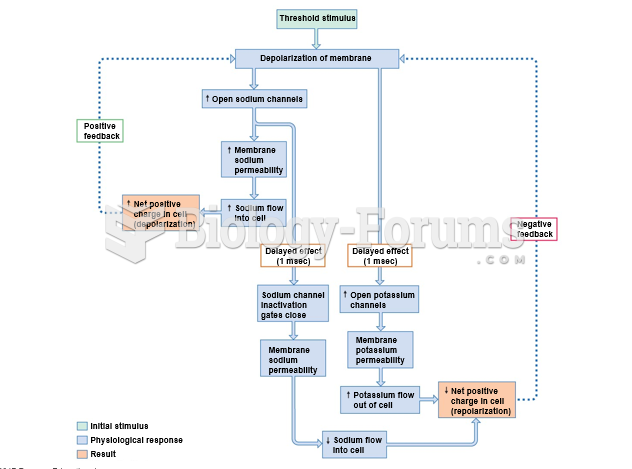
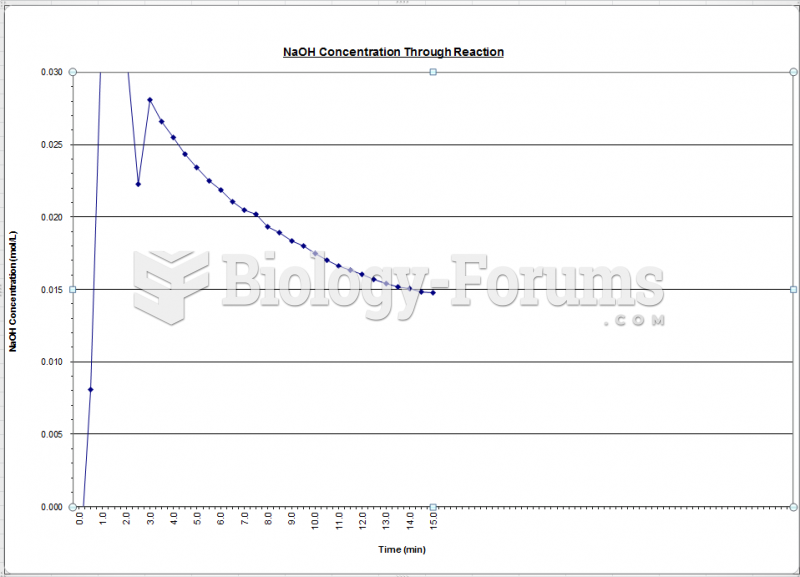
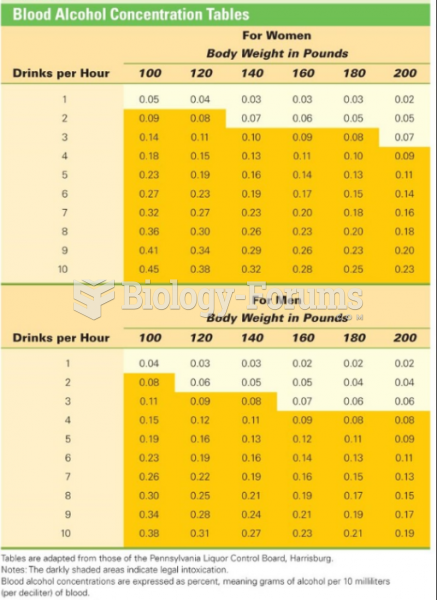
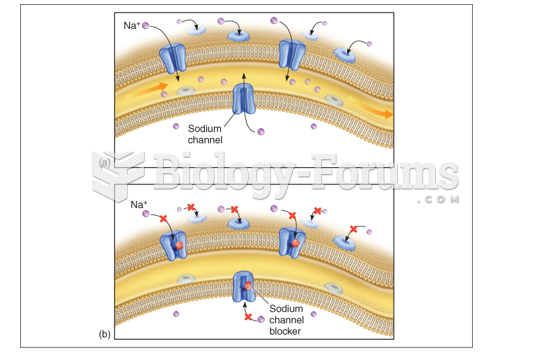
|
|
|
According to the FDA, adverse drug events harmed or killed approximately 1,200,000 people in the United States in the year 2015.
In most cases, kidneys can recover from almost complete loss of function, such as in acute kidney (renal) failure.
Famous people who died from poisoning or drug overdose include, Adolf Hitler, Socrates, Juan Ponce de Leon, Marilyn Monroe, Judy Garland, and John Belushi.
After a vasectomy, it takes about 12 ejaculations to clear out sperm that were already beyond the blocked area.
Hippocrates noted that blood separates into four differently colored liquids when removed from the body and examined: a pure red liquid mixed with white liquid material with a yellow-colored froth at the top and a black substance that settles underneath; he named these the four humors (for blood, phlegm, yellow bile, and black bile).