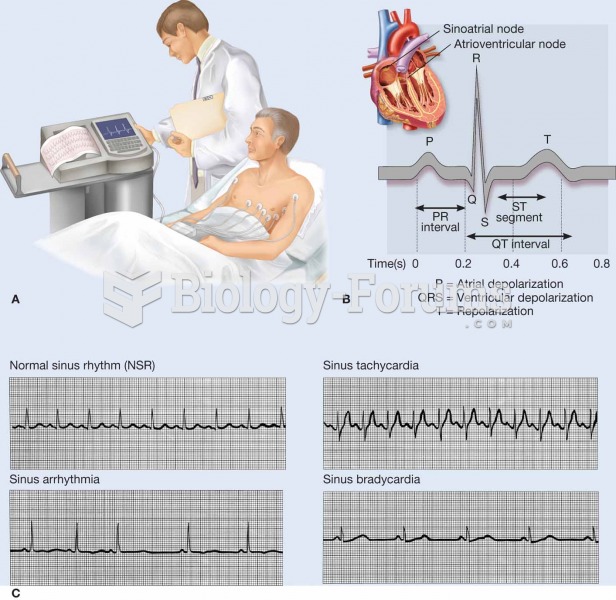
|
|
|
Human stomach acid is strong enough to dissolve small pieces of metal such as razor blades or staples.
Patients who have undergone chemotherapy for the treatment of cancer often complain of a lack of mental focus; memory loss; and a general diminution in abilities such as multitasking, attention span, and general mental agility.
Bacteria have been found alive in a lake buried one half mile under ice in Antarctica.
Hippocrates noted that blood separates into four differently colored liquids when removed from the body and examined: a pure red liquid mixed with white liquid material with a yellow-colored froth at the top and a black substance that settles underneath; he named these the four humors (for blood, phlegm, yellow bile, and black bile).
Recent studies have shown that the number of medication errors increases in relation to the number of orders that are verified per pharmacist, per work shift.